当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
X-ray crystal structure of embelin and its DFT scavenging of superoxide radical
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2017-08-28 , DOI: 10.1002/jcc.24915 Francesco Caruso 1 , Sarah Paumier 1 , Miriam Rossi 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2017-08-28 , DOI: 10.1002/jcc.24915 Francesco Caruso 1 , Sarah Paumier 1 , Miriam Rossi 1
Affiliation
|
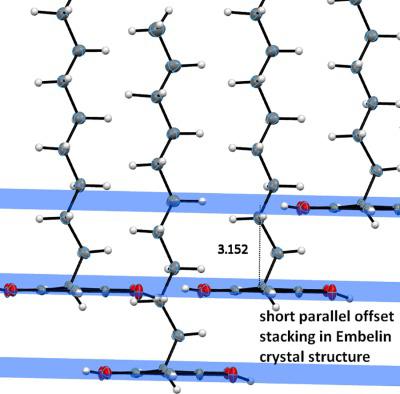
Embelin is a phytochemical component of tropical plants that have a long history of being used in ethnic pharmacology in various parts of the world, including Ayurdvedic and Chinese medicinal texts. Many modern studies confirm its promise as a medicinal compound. The X‐ray crystal structure determination of embelin shows a remarkably ordered alkyl chain and particularly strong pi–pi interactions for a nonaromatic system. The molecule has a torsion angle of 67° between the ring and the alkyl chain of the molecule and differs markedly from that seen when embelin is embedded in the plasminogen activator inhibitor‐1 (PAI‐1) binding site (almost planar—with about 10° torsion angle). This suggests that embelin's flexible structural skeleton can be useful in biological environment. Apart from this, its many biological activities likely depend on embelin's hydrophobic nonpolar tail that allows a variety of interactions. Computationally, we evaluated embelin's sequestering ability toward the superoxide radical and see that embelin executes this reaction in a novel manner. Namely, as shown by our DFT calculations, instead of releasing a H atom to the superoxide radical to form the anionic species O2H‐, embelin prefers to accept an electron from the superoxide radical, which then transforms into molecular oxygen, O2. © 2017 Wiley Periodicals, Inc.
中文翻译:

embelin的X射线晶体结构及其对超氧自由基的DFT清除
Embelin 是热带植物的一种植物化学成分,在世界各地的民族药理学中有着悠久的历史,包括阿育吠陀和中医文献。许多现代研究证实了它作为药用化合物的前景。embelin 的 X 射线晶体结构测定显示出非常有序的烷基链和特别强的非芳族体系的 pi-pi 相互作用。该分子在分子的环和烷基链之间具有 67° 的扭转角,与将 embelin 嵌入纤溶酶原激活剂抑制剂-1 (PAI-1) 结合位点(几乎是平面的,大约有 10 ° 扭转角)。这表明 embelin 的柔性结构骨架可用于生物环境。除此之外,它的许多生物活性可能取决于 embelin 的疏水性非极性尾部,它允许各种相互作用。通过计算,我们评估了 embelin 对超氧自由基的螯合能力,发现 embelin 以一种新颖的方式执行该反应。也就是说,正如我们的 DFT 计算所示,embelin 不是向超氧自由基释放 H 原子以形成阴离子物质 O2H-,而是更喜欢从超氧自由基接受一个电子,然后转化为分子氧 O2。© 2017 威利期刊公司。embelin 不是将 H 原子释放到超氧自由基以形成阴离子物质 O2H-,而是更喜欢接受来自超氧自由基的电子,然后转化为分子氧 O2。© 2017 威利期刊公司。embelin 不是将 H 原子释放到超氧自由基以形成阴离子物质 O2H-,而是更喜欢接受来自超氧自由基的电子,然后转化为分子氧 O2。© 2017 威利期刊公司。
更新日期:2017-08-28
中文翻译:

embelin的X射线晶体结构及其对超氧自由基的DFT清除
Embelin 是热带植物的一种植物化学成分,在世界各地的民族药理学中有着悠久的历史,包括阿育吠陀和中医文献。许多现代研究证实了它作为药用化合物的前景。embelin 的 X 射线晶体结构测定显示出非常有序的烷基链和特别强的非芳族体系的 pi-pi 相互作用。该分子在分子的环和烷基链之间具有 67° 的扭转角,与将 embelin 嵌入纤溶酶原激活剂抑制剂-1 (PAI-1) 结合位点(几乎是平面的,大约有 10 ° 扭转角)。这表明 embelin 的柔性结构骨架可用于生物环境。除此之外,它的许多生物活性可能取决于 embelin 的疏水性非极性尾部,它允许各种相互作用。通过计算,我们评估了 embelin 对超氧自由基的螯合能力,发现 embelin 以一种新颖的方式执行该反应。也就是说,正如我们的 DFT 计算所示,embelin 不是向超氧自由基释放 H 原子以形成阴离子物质 O2H-,而是更喜欢从超氧自由基接受一个电子,然后转化为分子氧 O2。© 2017 威利期刊公司。embelin 不是将 H 原子释放到超氧自由基以形成阴离子物质 O2H-,而是更喜欢接受来自超氧自由基的电子,然后转化为分子氧 O2。© 2017 威利期刊公司。embelin 不是将 H 原子释放到超氧自由基以形成阴离子物质 O2H-,而是更喜欢接受来自超氧自由基的电子,然后转化为分子氧 O2。© 2017 威利期刊公司。











































 京公网安备 11010802027423号
京公网安备 11010802027423号