当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical Protein Synthesis with the α-Ketoacid–Hydroxylamine Ligation
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2017-08-29 00:00:00 , DOI: 10.1021/acs.accounts.7b00277 Jeffrey W. Bode 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2017-08-29 00:00:00 , DOI: 10.1021/acs.accounts.7b00277 Jeffrey W. Bode 1
Affiliation
|
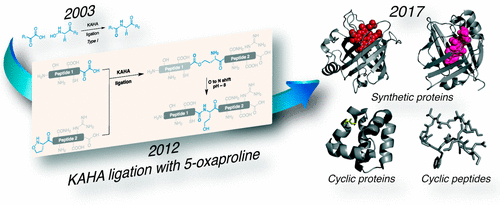
The coupling of an α-ketoacid and a hydroxylamine (KAHA ligation) affords amide bonds under aqueous, acidic conditions without the need for protecting groups or coupling agents. Translating this finding into a general approach to chemical protein synthesis required the identification of methods to incorporate the key functional groups into unprotected peptide segments—ideally using well-established Fmoc solid-phase peptide synthesis protocols. A decade of effort has now led to robust, convenient methods for preparing peptides bearing free or masked C-terminal α-ketoacids and N-terminal hydroxylamines. The facile synthesis of the segments and the aqueous, acidic conditions of the KAHA ligation make it ideal for the construction of small proteins (up to 200 residues), including SUMO and related modifier proteins, betatrophin and other protein hormones, nitrophorin 4, S100A4, and the cyclic protein AS-48.
中文翻译:

α-酮酸-羟胺连接的化学蛋白质合成
α-酮酸和羟胺的偶联(KAHA连接)可在水性酸性条件下提供酰胺键,而无需保护基团或偶联剂。将这一发现转化为化学蛋白质合成的一般方法,需要确定将关键官能团整合到未保护的肽段中的方法-理想情况下,使用成熟的Fmoc固相肽合成方案。十年的努力现已导致一种健壮,方便的方法,用于制备带有游离或掩蔽的C端α-酮酸和N端羟胺的肽。片段的容易合成以及KAHA连接的水性酸性条件使其非常适合构建小蛋白(最多200个残基),包括SUMO和相关修饰蛋白,
更新日期:2017-08-29
中文翻译:

α-酮酸-羟胺连接的化学蛋白质合成
α-酮酸和羟胺的偶联(KAHA连接)可在水性酸性条件下提供酰胺键,而无需保护基团或偶联剂。将这一发现转化为化学蛋白质合成的一般方法,需要确定将关键官能团整合到未保护的肽段中的方法-理想情况下,使用成熟的Fmoc固相肽合成方案。十年的努力现已导致一种健壮,方便的方法,用于制备带有游离或掩蔽的C端α-酮酸和N端羟胺的肽。片段的容易合成以及KAHA连接的水性酸性条件使其非常适合构建小蛋白(最多200个残基),包括SUMO和相关修饰蛋白,











































 京公网安备 11010802027423号
京公网安备 11010802027423号