JAMA Oncology ( IF 22.5 ) Pub Date : 2018-01-01 , DOI: 10.1001/jamaoncol.2017.2752 Louise Emilsson 1, 2, 3 , Xabier García-Albéniz 1 , Roger W Logan 1 , Ellen C Caniglia 1 , Mette Kalager 1, 2 , Miguel A Hernán 1, 4, 5
|
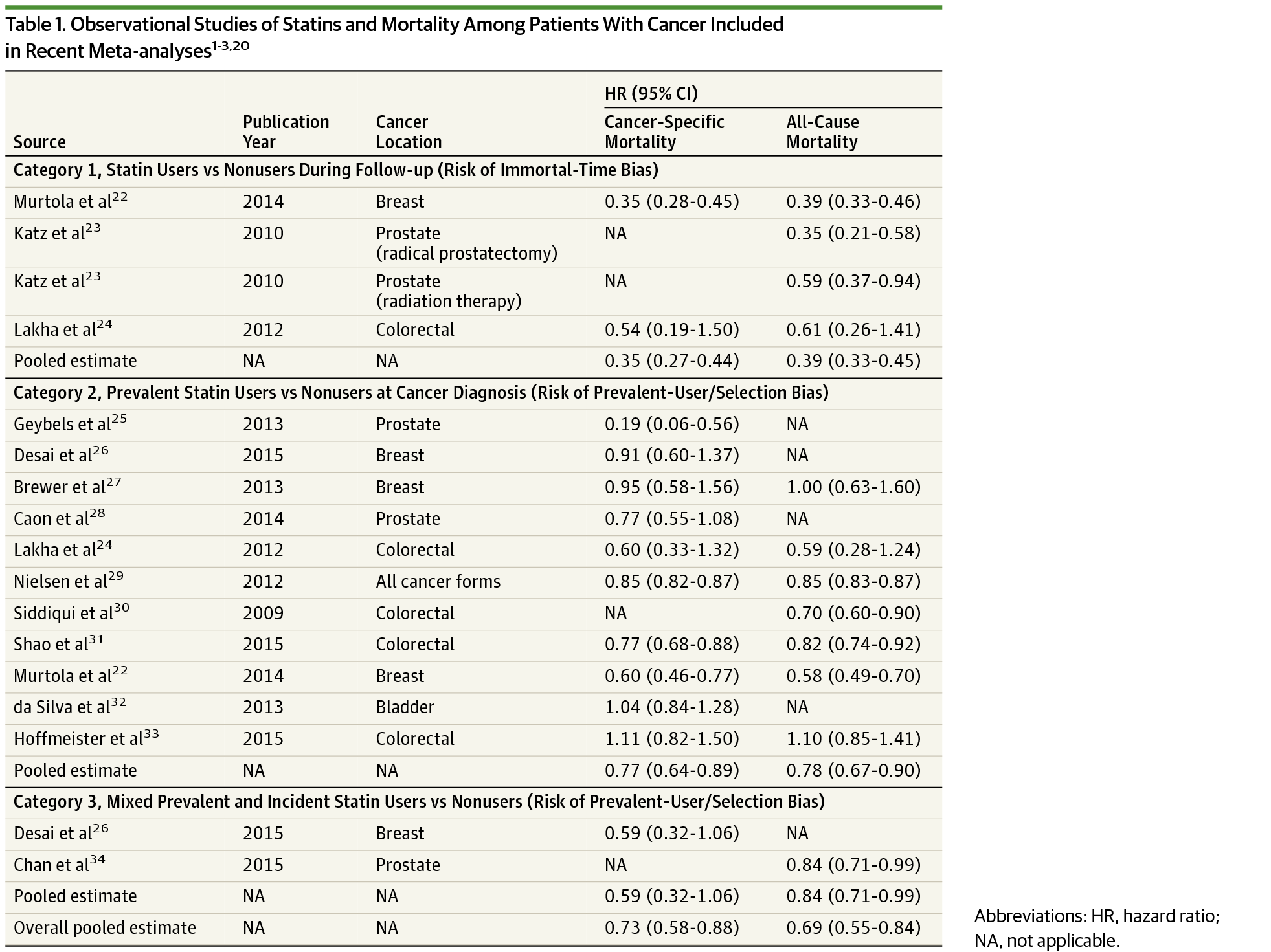
Importance Patients with cancer who use statins appear to have a substantially better survival than nonusers in observational studies. However, this inverse association between statin use and mortality may be due to selection bias and immortal-time bias.
Objective To emulate a randomized trial of statin therapy initiation that is free of selection bias and immortal-time bias.
Design, Setting, and Participants We used observational data on 17 372 patients with cancer from the Surveillance, Epidemiology, and End Results (SEER)-Medicare database (2007-2009) with complete follow-up until 2011. The SEER-Medicare database links 17 US cancer registries and claims files from Medicare and Medicaid in 12 US states. We included individuals with a new diagnosis of colorectal, breast, prostate, or bladder cancer who had not been prescribed statins for at least 6 months before the cancer diagnosis. Individuals were duplicated, and each replicate was assigned to either the strategy “statin therapy initiation within 6 months after diagnosis” or “no statin therapy initiation.” Replicates were censored when they stopped following their assigned strategy, and the potential selection bias was adjusted for via inverse-probability weighting. Hazard ratios (HRs), cumulative incidences, and risk differences were calculated for all-cause mortality and cancer-specific mortality. We then compared our estimates with those obtained using the same analytic approaches used in previous observational studies.
Exposures Statin therapy initiation within 6 months after cancer diagnosis.
Main Outcomes and Measures Cancer-specific and all-cause mortality using SEER-Medicare data and data from previous studies.
Results Of the 17 372 patients whose data were analyzed, 8440 (49%) were men, and 8932 (51%) were women (mean [SD] age, 76.4 [7.4] years; range, 66-115 years). The adjusted HR (95% CI) comparing statin therapy initiation vs no initiation was 1.00 (0.88-1.15) for cancer-specific mortality and 1.07 (0.93-1.21) for overall mortality. Cumulative incidence curves for both groups were almost overlapping (the risk difference never exceeded 0.8%). In contrast, the methods used by prior studies resulted in an inverse association between statin use and mortality (pooled hazard ratio 0.69).
Conclusion and Relevance After using methods that are not susceptible to selection bias from prevalent users and to immortal time bias, we found that initiation of therapy with statins within 6 months after cancer diagnosis did not appear to improve 3-year cancer-specific or overall survival.
中文翻译:

检查癌症患者他汀类药物治疗和生存研究的偏倚
重要性 在观察性研究中,使用他汀类药物的癌症患者似乎比不使用他汀类药物的患者有更好的生存率。然而,他汀类药物使用与死亡率之间的这种负相关可能是由于选择偏差和永生时间偏差。
目的 模拟一项没有选择偏倚和永生时间偏倚的他汀类药物治疗开始的随机试验。
设计、设置和参与者 我们使用了来自监测、流行病学和最终结果 (SEER)-Medicare 数据库 (2007-2009) 的 17 372 名癌症患者的观察数据,并进行了完整的随访至 2011 年。SEER-Medicare 数据库链接了 17 个美国癌症登记和索赔来自美国 12 个州的医疗保险和医疗补助的文件。我们纳入了新诊断为结直肠癌、乳腺癌、前列腺癌或膀胱癌的个体,这些个体在癌症诊断前至少 6 个月未服用他汀类药物。个体被重复,每个重复被分配到策略“诊断后 6 个月内开始他汀类药物治疗”或“不开始他汀类药物治疗”。当他们停止遵循指定的策略时,对复制品进行审查,并通过逆概率加权调整潜在的选择偏差。危险比(HR),计算了全因死亡率和癌症特异性死亡率的累积发病率和风险差异。然后,我们将我们的估计与使用先前观察研究中使用的相同分析方法获得的估计进行了比较。
癌症 诊断后 6 个月内开始接受他汀类药物治疗。
主要结果和测量 使用 SEER-Medicare 数据和先前研究的数据的癌症特异性和全因死亡率。
结果 在分析数据的 17 372 名患者中,8440 名(49%)为男性,8932 名(51%)为女性(平均 [SD] 年龄,76.4 [7.4] 岁;范围,66-115 岁)。比较他汀类药物治疗开始与未开始治疗的调整后 HR (95% CI) 为癌症特异性死亡率 1.00 (0.88-1.15) 和总死亡率 1.07 (0.93-1.21)。两组的累积发病率曲线几乎重叠(风险差异从未超过 0.8%)。相比之下,先前研究使用的方法导致他汀类药物的使用与死亡率之间存在负相关(汇总风险比 0.69)。
结 _ .











































 京公网安备 11010802027423号
京公网安备 11010802027423号