JAMA Oncology ( IF 22.5 ) Pub Date : 2018-01-01 , DOI: 10.1001/jamaoncol.2017.2391 Alexander N Shoushtari 1, 2 , Claire F Friedman 1, 2 , Pedram Navid-Azarbaijani 1, 2 , Michael A Postow 1, 2 , Margaret K Callahan 1, 2 , Parisa Momtaz 1, 2 , Katherine S Panageas 3 , Jedd D Wolchok 1, 2 , Paul B Chapman 1, 2
|
Importance Nivolumab plus ipilimumab (nivo + ipi) is a standard treatment of advanced melanoma. Two randomized trials describe high objective response rates by Response Evaluation Criteria in Solid Tumors. The trials assessed toxic effects using the Common Terminology Criteria for Adverse Events (CTCAE), which may underestimate incidence of clinically significant immune-related adverse events (AEs).
Objective To describe detailed toxic effects and time to treatment failure of patients with melanoma treated with nivo + ipi in a prospective cohort.
Design, Setting, and Participants A cohort of 64 adults with advanced or unresectable melanoma were examined at a single tertiary cancer and enrolled in an expanded access program of nivo + ipi conducted from December 2014 to January 2016.
Interventions Intravenous nivolumab (1 mg/kg) and ipilimumab (3 mg/kg) administered every 3 weeks for up to 4 doses, followed by nivolumab (3 mg/kg) every 2 weeks or pembrolizumab (2 mg/kg) every 3 weeks until unacceptable toxic effects, disease progression, or complete response.
Main Outcomes and Measures Clinically significant immune-related AEs were defined as CTCAE grade 2 or higher or any immune-related AEs requiring systemic steroids. Time to treatment failure was defined as the interval between initiating therapy and the earliest of clinical progression, new locally directed or systemic treatment other than anti–programmed cell death 1 protein (anti–PD-1) monotherapy, or death.
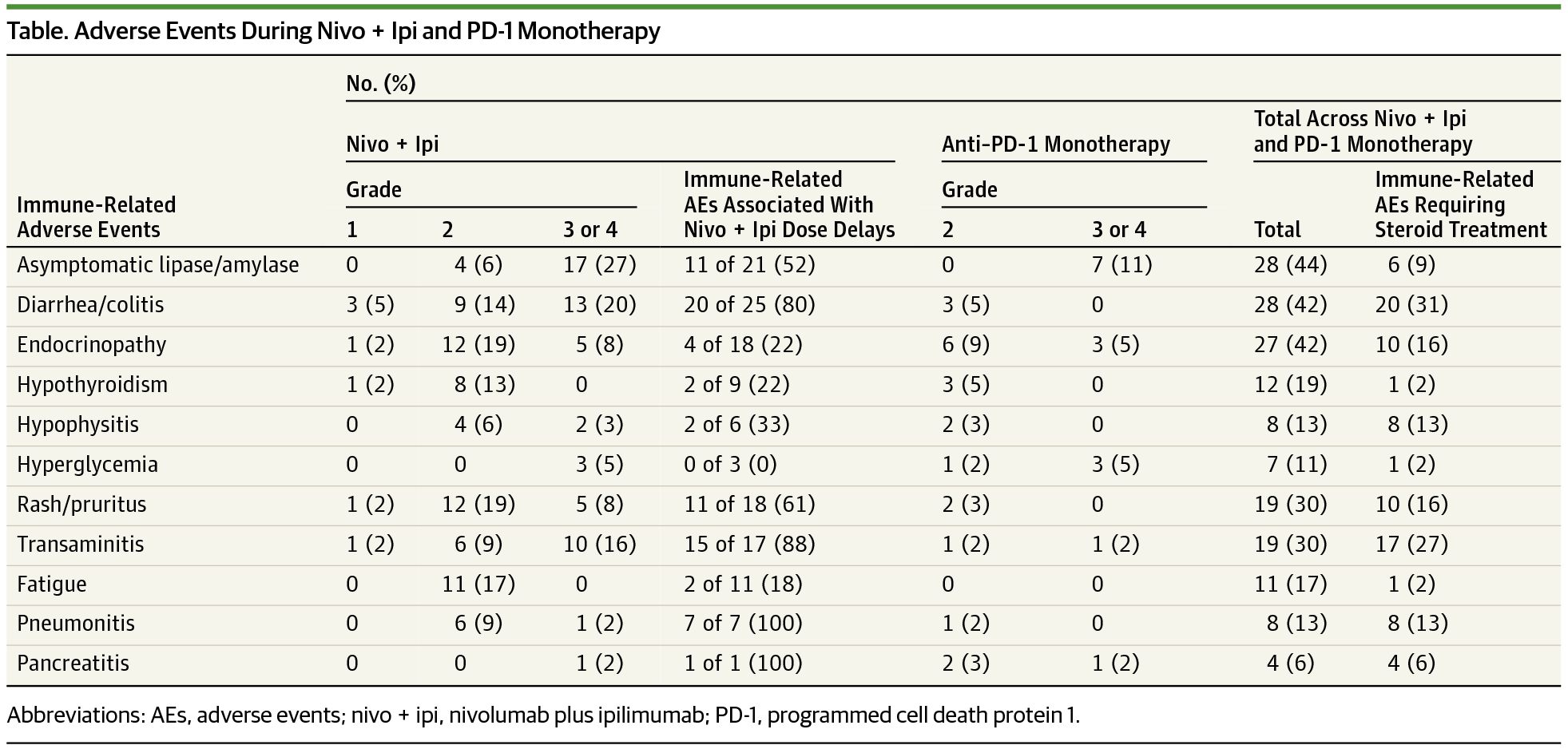
Results Overall 64 adults with advanced or unresectable melanoma were enrolled (male to female ratio, 1:1; median [range] age, 56 [22-82] years); 25 patients (39%) received all 4 doses of nivo + ipi, and 31 patients (48%) received no maintenance anti–PD-1 therapy. Most who discontinued treatment (n = 31 [80%]) stopped because of toxic effects. Among those patients who were progression free at 12 weeks, time to treatment failure was similar between those who did or did not modify therapy for toxic effects. Fifty-eight patients (91%) had a clinically significant immune-related AE (median, 2/patient), and 46 patients (72%) required systemic steroids. Infliximab or mycophenolate was required in 16 patients (25%) for steroid-refractory immune-related AEs. Seven patients (11%) developed hyperglycemia, 32 patients (50%) had an emergency department visit, and 23 patients (36%) required a hospital admission related to an immune-related AE. Four of 31 patients (13%) who stopped combination therapy early for toxic effects developed a new, clinically significant immune-related AE more than 16 weeks after the last treatment.
Conclusions and Relevance We observed a 91% incidence of clinically significant immune-related AEs leading to frequent emergency department visits, hospitalizations, and systemic immunosuppression. Immuno-oncology trials should routinely report these metrics. Most patients do not tolerate 4 doses of nivo + ipi; however, 4 doses may not be required for clinical benefit.
中文翻译:

测量纳武单抗加伊匹单抗治疗黑色素瘤的毒性作用和治疗失败时间
重要性 纳武单抗联合易普利姆玛 (nivo + ipi) 是晚期黑色素瘤的标准治疗方法。两项随机试验描述了实体瘤疗效评估标准的高客观缓解率。这些试验使用不良事件通用术语标准(CTCAE)评估毒性作用,这可能低估了临床上显着的免疫相关不良事件(AE)的发生率。
目的 描述前瞻性队列中接受 nivo + ipi 治疗的黑色素瘤患者的详细毒性反应和治疗失败时间。
设计、背景和参与者 对 64 名患有晚期或不可切除黑色素瘤的成年人进行了单次三级癌症检查,并参加了 2014 年 12 月至 2016 年 1 月进行的 nivo + ipi 扩大访问计划。
干预措施 每 3 周静脉注射纳武单抗 (1 mg/kg) 和易普利姆玛 (3 mg/kg),最多 4 剂,然后每 2 周注射纳武单抗 (3 mg/kg) 或每 3 周注射派姆单抗 (2 mg/kg)直到出现不可接受的毒性作用、疾病进展或完全缓解。
主要结果和措施 临床上显着的免疫相关 AE 定义为 CTCAE 2 级或更高级别或任何需要全身类固醇的免疫相关 AE。治疗失败时间定义为开始治疗与最早出现临床进展、除抗程序性细胞死亡1蛋白(抗PD-1)单一疗法外的新的局部定向或全身治疗或死亡之间的时间间隔。
结果 总共招募了 64 名患有晚期或不可切除黑色素瘤的成人(男女比例为 1:1;中位年龄 [范围] 56 [22-82] 岁);25 名患者 (39%) 接受了全部 4 剂 nivo + ipi,31 名患者 (48%) 未接受维持性抗 PD-1 治疗。大多数停止治疗的人 (n = 31 [80%]) 由于毒性作用而停止。在 12 周时无进展的患者中,治疗失败的时间在那些因毒性反应而改变治疗或未改变治疗的患者之间相似。58 名患者 (91%) 出现临床显着的免疫相关 AE(中位数,2 名/患者),46 名患者 (72%) 需要全身类固醇。16 名患者 (25%) 需要使用英夫利昔单抗或麦考酚酯来治疗类固醇难治性免疫相关 AE。7 名患者 (11%) 出现高血糖,32 名患者 (50%) 前往急诊室就诊,23 名患者 (36%) 因免疫相关 AE 需要入院。因毒性作用而提前停止联合治疗的 31 名患者中,有 4 名 (13%) 在最后一次治疗后超过 16 周出现了新的、具有临床意义的免疫相关 AE。
结论和相关性 我们观察到临床显着的免疫相关 AE 的发生率为 91%,导致频繁急诊就诊、住院治疗和全身免疫抑制。免疫肿瘤学试验应定期报告这些指标。大多数患者不能耐受 4 剂 nivo + ipi;然而,可能不需要 4 剂才能获得临床益处。











































 京公网安备 11010802027423号
京公网安备 11010802027423号