Journal of Photochemistry and Photobiology C: Photochemistry Reviews ( IF 12.8 ) Pub Date : 2016-06-03 , DOI: 10.1016/j.jphotochemrev.2016.05.003 Anton J. Stasyuk , Piotr J. Cywiński , Daniel T. Gryko
|
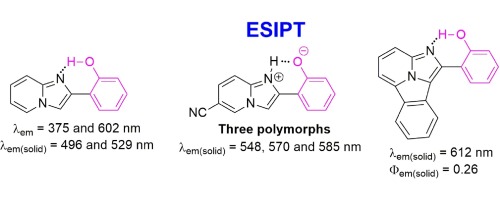
The key observation made by Acuña revealed the unusual optical properties of previously unknown 2-(2′-hydroxyphenyl)imidazo[1,2-a]pyridines (HPIPs). Although structurally similar to 2′-(2′-hydroxyphenyl)benzoxazoles and 2-(2′-hydroxyphenyl)benzimidazoles, 2-(2′-hydroxyphenyl)imidazo[1,2-a]pyridines exhibit strikingly different optical properties, especially in the solid state. The luminescence color of HPIPs depends on the polymorphic form and can be tailored from green to red. The inability to assume the neutral keto tautomer is their other distinct characteristic, setting them apart from analogous azoles and their benzoanalogs. For these azoles and related luminescent compounds, the dual emission in polar solvents is usually observed, dominated by excited-state intramolecular proton transfer (ESIPT) fluorescence. However, for 2-(2′-hydroxyphenyl)imidazo[1,2-a]pyridines, clear ESIPT emission exists only in non-polar solvents and it is very weak, while in polar and especially protic solvents, strong emission from the Franck-Condon state is exclusively observed. The solvent-related effect is attributed to the tendency to form strong intramolecular hydrogen bonds in the solid state and in non-polar solvents, while hydrogen bond formation is much weaker in polar, and especially, in protic solvents. The present study focuses on HPIPs and demonstrates that ESIPT process is a promising mechanism for packing-directed luminescence control and offers a novel design concept for tunable organic luminescent solids. This review presents not only optical properties in various solutions, but also in the solid state, and it is supported by a synthetic overview, computational studies, and the description of π-expanded derivatives.
中文翻译:

2'-(2'-羟苯基)咪唑并[1,2- a ]吡啶的激发态分子内质子转移
阿库尼亚(Acuña)所作的关键观察揭示了以前未知的2-(2'-羟苯基)咪唑并[1,2- a ]吡啶(HPIPs)的不同寻常的光学性质。尽管在结构上类似于2'-(2'-羟基苯基)苯并恶唑和2-(2'-羟基苯基)苯并咪唑,但是2-(2'-羟基苯基)咪唑[1,2- a对吡啶显示出显着不同的光学性质,尤其是在固态下。HPIP的发光颜色取决于多态形式,可以从绿色变为红色。不能假定中性酮互变异构体是它们的另一个独特特征,使它们与类似的唑类及其苯并类似物区分开。对于这些唑类和相关的发光化合物,通常在极性溶剂中观察到双重发射,主要受激发态分子内质子转移(ESIPT)荧光的影响。然而,对于2-(2'-羟基苯基)咪唑并[1,2- a]吡啶,仅在非极性溶剂中存在清晰的ESIPT发射,并且非常弱,而在极性溶剂(尤其是质子溶剂)中,仅观察到来自Franck-Condon状态的强发射。与溶剂有关的作用归因于在固态和非极性溶剂中倾向于形成强分子内氢键的趋势,而在极性尤其是在质子溶剂中氢键的形成要弱得多。本研究的重点是HPIP,并证明ESIPT工艺是用于包装定向发光控制的一种有前途的机制,并为可调谐有机发光固体提供了新颖的设计概念。这篇评论不仅介绍了各种溶液的光学性质,而且还介绍了固态的光学性质,并且得到了综述,计算研究,











































 京公网安备 11010802027423号
京公网安备 11010802027423号