JAMA Oncology ( IF 22.5 ) Pub Date : 2018-01-01 , DOI: 10.1001/jamaoncol.2017.2303 Mary Feng 1, 2 , Krithika Suresh 3 , Matthew J Schipper 1, 3 , Latifa Bazzi 1 , Edgar Ben-Josef 4 , Martha M Matuszak 1 , Neehar D Parikh 5 , Theodore H Welling 6 , Daniel Normolle 7 , Randall K Ten Haken 1 , Theodore S Lawrence 1
|
Importance Patients with preexisting liver dysfunction could benefit the most from personalized therapy for liver tumors to balance maximal tumor control and minimal risk of liver failure. We designed an individualized adaptive trial testing the hypothesis that adapting treatment based on change in liver function could optimize the therapeutic index for each patient.
Objective To characterize the safety and efficacy of individualized adaptive stereotactic body radiotherapy (SRBT) for liver tumors in patients who have preexisting liver dysfunction.
Design, Setting, and Participants From 2010 to 2014, 90 patients with intrahepatic cancer treated with prior liver-directed therapy were enrolled in this large phase 2, single-arm, clinical trial at an academic medical center. All patients had at least 1 year of potential follow-up.
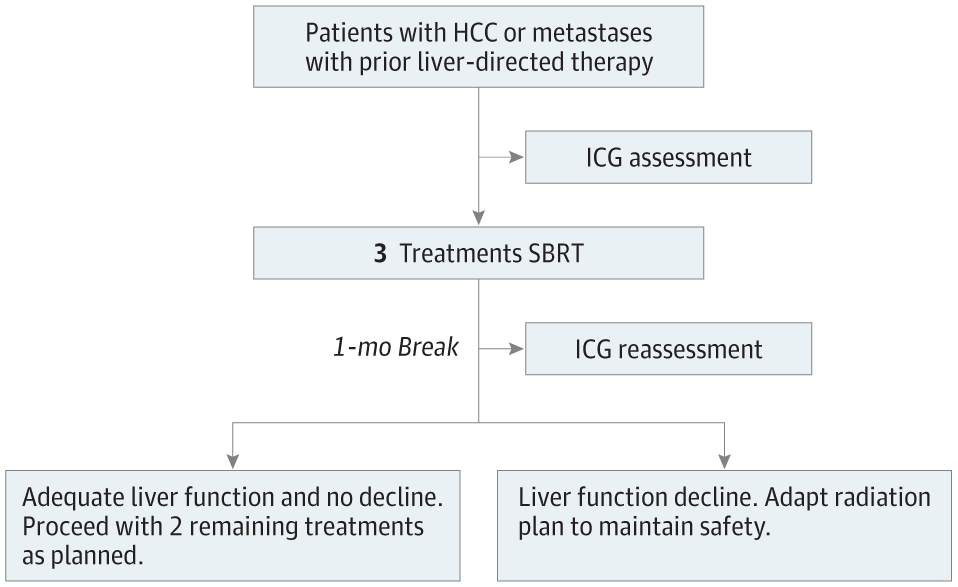
Interventions Using indocyanine green retention at 15 minutes (ICGR15) as a direct biomarker of liver function and a Bayesian adaptive model, planned SBRT was individually modified midway through the course of therapy to maintain liver function after the complete course.
Main Outcomes and Measures The primary outcome was local control; the secondary outcome was safety and overall survival.
Results Patients were 34 to 85 years of age, and 70% (63) were male. Ninety patients (69 [77%] with hepatocellular carcinoma, 4 [4%] with intrahepatic cholangiocarcinoma, and 17 [19%] with metastatic) received treatment to 116 tumors. Sixty-two patients (69%) had cirrhosis, 21 (23%) were Child-Pugh (CP) grade B. The median tumor size was 3 cm; 16 patients (18%) had portal vein involvement. Sixty-two (69%) received all 5 fractions (47 full dose, 15 dose-reduced owing to rising ICGR15). Treatment was well tolerated, with a lower than expected complication rate without adaptation: 6 (7%) experienced a 2-point decline in CP 6 months post-SBRT. The 1- and 2-year local control rates were 99% (95% CI, 97%-100%) and 95% (95% CI, 91%-99%), respectively.
Conclusions and Relevance We demonstrated that the treatment strategy of individualized adaptive therapy based on a direct biomarker of liver function can be used to achieve both high rates of local control and a high degree of safety without sacrificing either. Individualized adaptive radiotherapy may represent a new treatment paradigm in which dose is based on individual, rather than population-based, tolerance to treatment.
Trial Registration clinicaltrials.gov Identifier: NCT01522937
中文翻译:

个体化自适应立体定向放射治疗肝损伤高危患者的肝肿瘤A 2 期临床试验
重要性 预先存在肝功能障碍的患者可以从肝肿瘤的个体化治疗中受益最大,以平衡最大的肿瘤控制和肝衰竭的最小风险。我们设计了一项个性化的适应性试验,测试基于肝功能变化调整治疗可以优化每位患者的治疗指数的假设。
目的 探讨个体化适应性立体定向体部放射治疗(SRBT)治疗既往肝功能不全患者肝脏肿瘤的安全性和有效性。
设计、设置和参与者 从 2010 年到 2014 年,90 名接受过肝脏定向治疗的肝内癌患者参加了在学术医疗中心进行的这项大型 2 期单臂临床试验。所有患者都进行了至少 1 年的潜在随访。
干预 使用 15 分钟吲哚菁绿保留 (ICGR15) 作为肝功能的直接生物标志物和贝叶斯自适应模型,计划的 SBRT 在治疗过程的中途单独修改,以在整个疗程后维持肝功能。
主要结果和措施 主要结果是局部控制;次要结果是安全性和总生存期。
结果 患者年龄34~85岁,70%(63)为男性。90 名患者(69 名 [77%] 患有肝细胞癌,4 名 [4%] 患有肝内胆管癌,17 名 [19%] 患有转移性)接受了 116 个肿瘤的治疗。62 名患者 (69%) 患有肝硬化,21 名 (23%) 为 Child-Pugh (CP) B 级。中位肿瘤大小为 3 cm;16 名患者 (18%) 有门静脉受累。62 人 (69%) 接受了所有 5 次分割(47 人全剂量,15 人因 ICGR15 升高而减少剂量)。治疗耐受性良好,未适应的并发症发生率低于预期:6 (7%) 例患者在 SBRT 后 6 个月内 CP 下降 2 点。1年和2年局部控制率分别为99%(95% CI,97%-100%)和95%(95% CI,91%-99%)。
结论和相关性 我们证明了基于肝功能直接生物标志物的个体化适应性治疗的治疗策略可用于实现高局部控制率和高度安全性,而不会牺牲两者。个体化适应性放射治疗可能代表一种新的治疗范式,其中剂量基于个体而不是基于人群的治疗耐受性。
试验注册 临床试验.gov 标识符:NCT01522937











































 京公网安备 11010802027423号
京公网安备 11010802027423号