当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ribosome Rescue Inhibitors Kill Actively Growing and Nonreplicating Persister Mycobacterium tuberculosis Cells
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2017-08-07 00:00:00 , DOI: 10.1021/acsinfecdis.7b00028 John N. Alumasa 1 , Paolo S. Manzanillo 2 , Nicholas D. Peterson 3 , Tricia Lundrigan 2 , Anthony D. Baughn 3 , Jeffery S. Cox 2 , Kenneth C. Keiler 1
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2017-08-07 00:00:00 , DOI: 10.1021/acsinfecdis.7b00028 John N. Alumasa 1 , Paolo S. Manzanillo 2 , Nicholas D. Peterson 3 , Tricia Lundrigan 2 , Anthony D. Baughn 3 , Jeffery S. Cox 2 , Kenneth C. Keiler 1
Affiliation
|
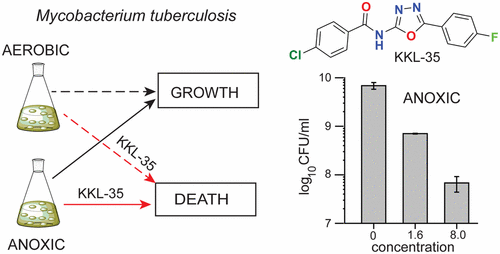
The emergence of Mycobacterium tuberculosis (MTB) strains that are resistant to most or all available antibiotics has created a severe problem for treating tuberculosis and has spurred a quest for new antibiotic targets. Here, we demonstrate that trans-translation is essential for growth of MTB and is a viable target for development of antituberculosis drugs. We also show that an inhibitor of trans-translation, KKL-35, is bactericidal against MTB under both aerobic and anoxic conditions. Biochemical experiments show that this compound targets helix 89 of the 23S rRNA. In silico molecular docking predicts a binding pocket for KKL-35 adjacent to the peptidyl-transfer center in a region not targeted by conventional antibiotics. Computational solvent mapping suggests that this pocket is a druggable hot spot for small molecule binding. Collectively, our findings reveal a new target for antituberculosis drug development and provide critical insight on the mechanism of antibacterial action for KKL-35 and related 1,3,4-oxadiazole benzamides.
中文翻译:

核糖体拯救抑制剂杀死活跃的和非复制性的持久性结核分枝杆菌细胞
对大多数或所有可用抗生素具有抗性的结核分枝杆菌(MTB)菌株的出现给治疗结核病带来了严重的问题,并刺激了对新抗生素靶标的需求。在这里,我们证明了反式翻译对于MTB的生长至关重要,并且是抗结核药物开发的可行目标。我们还显示,反翻译抑制剂KKL-35在有氧和缺氧条件下均对MTB具有杀菌作用。生化实验表明,该化合物靶向23S rRNA的螺旋89。在计算机上分子对接预测了在传统抗生素未靶向的区域中,邻近肽基转移中心的KKL-35的结合口袋。计算溶剂作图表明,该口袋是小分子结合的可治疗热点。总的来说,我们的发现揭示了抗结核药物开发的新目标,并为KKL-35和相关的1,3,4-恶二唑苯甲酰胺的抗菌作用机理提供了重要的见识。
更新日期:2017-08-07
中文翻译:

核糖体拯救抑制剂杀死活跃的和非复制性的持久性结核分枝杆菌细胞
对大多数或所有可用抗生素具有抗性的结核分枝杆菌(MTB)菌株的出现给治疗结核病带来了严重的问题,并刺激了对新抗生素靶标的需求。在这里,我们证明了反式翻译对于MTB的生长至关重要,并且是抗结核药物开发的可行目标。我们还显示,反翻译抑制剂KKL-35在有氧和缺氧条件下均对MTB具有杀菌作用。生化实验表明,该化合物靶向23S rRNA的螺旋89。在计算机上分子对接预测了在传统抗生素未靶向的区域中,邻近肽基转移中心的KKL-35的结合口袋。计算溶剂作图表明,该口袋是小分子结合的可治疗热点。总的来说,我们的发现揭示了抗结核药物开发的新目标,并为KKL-35和相关的1,3,4-恶二唑苯甲酰胺的抗菌作用机理提供了重要的见识。



























 京公网安备 11010802027423号
京公网安备 11010802027423号