当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigating the Interaction of Octapeptin A3 with Model Bacterial Membranes
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2017-07-11 00:00:00 , DOI: 10.1021/acsinfecdis.7b00065 Mei-Ling Han 1 , Hsin-Hui Shen , Karl A. Hansford 2 , Elena K. Schneider 1 , Sivashangarie Sivanesan 1 , Kade D. Roberts 1 , Philip E. Thompson 1 , Anton P. Le Brun 3 , Yan Zhu 1 , Marc-Antoine Sani 4 , Frances Separovic 4 , Mark A. T. Blaskovich 2 , Mark A. Baker 5 , Samuel M. Moskowitz 6 , Matthew A. Cooper 2 , Jian Li , Tony Velkov 1
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2017-07-11 00:00:00 , DOI: 10.1021/acsinfecdis.7b00065 Mei-Ling Han 1 , Hsin-Hui Shen , Karl A. Hansford 2 , Elena K. Schneider 1 , Sivashangarie Sivanesan 1 , Kade D. Roberts 1 , Philip E. Thompson 1 , Anton P. Le Brun 3 , Yan Zhu 1 , Marc-Antoine Sani 4 , Frances Separovic 4 , Mark A. T. Blaskovich 2 , Mark A. Baker 5 , Samuel M. Moskowitz 6 , Matthew A. Cooper 2 , Jian Li , Tony Velkov 1
Affiliation
|
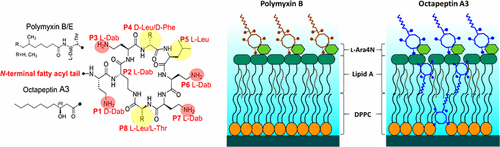
Octapeptins are cyclic lipopeptides with a broader spectrum of activity against fungi and polymyxin-resistant Gram-negative and Gram-positive bacteria. In the present study, we investigated the interaction of octapeptin A3 with asymmetric outer membrane models of Gram-negative pathogen Pseudomonas aeruginosa using neutron reflectometry, together with fluorimetric and calorimetry methods. For the first time, our neutron reflectometry results reveal that the interaction of octapeptin A3 with the Gram-negative outer membrane involves an initial transient polar interaction with the phospholipid and lipid A headgroups, followed by the penetration of the entire octapeptin molecule into the fatty acyl core of the outer membrane. This mechanism contrasts with that of polymyxin B, which specifically targets lipid A, whereas octapeptins appear to target both lipid A and phospholipids. Furthermore, the mechanism of octapeptins does not appear to be highly dependent on an initial complementary electrostatic interaction with lipid A, which accounts for their ability to bind to lipid A of polymyxin-resistant Gram-negative bacteria that is modified with cationic moieties that act to electrostatically repel the cationic polymyxin molecule. The presented findings shed new light on the mechanism whereby octapeptins penetrate the outer membrane of polymyxin-resistant Gram-negative pathogens and highlight their potential as candidates for development as new antibiotics against problematic multi-drug-resistant pathogens.
中文翻译:

研究Octepteptin A3与模型细菌膜的相互作用
八肽是环状脂肽,具有抗真菌和抗多粘菌性革兰氏阴性和革兰氏阳性细菌的广谱活性。在本研究中,我们调查了八肽素A3与革兰氏阴性病菌铜绿假单胞菌的不对称外膜模型的相互作用。使用中子反射法,以及荧光法和量热法。我们的中子反射测量结果首次显示,八肽素A3与革兰氏阴性外膜的相互作用涉及与磷脂和脂质A头基团的初始瞬时极性相互作用,然后整个八肽素分子渗透到脂肪酰基中外膜的核心。该机制与特异性针对脂质A的多粘菌素B相反,而八肽似乎同时靶向脂质A和磷脂。此外,八肽的机制似乎并不高度依赖与脂质A的初始互补性静电相互作用,这说明了它们与抗多粘菌素的革兰氏阴性细菌的脂质A结合的能力,该细菌被阳离子部分修饰,这些阳离子部分可静电排斥阳离子多粘菌素分子。提出的发现为八肽蛋白渗透多粘菌抗性革兰氏阴性病原体的外膜提供了新的机制,并突出了它们作为抗病多药耐药性病原体的新抗生素开发的潜力。
更新日期:2017-07-11
中文翻译:

研究Octepteptin A3与模型细菌膜的相互作用
八肽是环状脂肽,具有抗真菌和抗多粘菌性革兰氏阴性和革兰氏阳性细菌的广谱活性。在本研究中,我们调查了八肽素A3与革兰氏阴性病菌铜绿假单胞菌的不对称外膜模型的相互作用。使用中子反射法,以及荧光法和量热法。我们的中子反射测量结果首次显示,八肽素A3与革兰氏阴性外膜的相互作用涉及与磷脂和脂质A头基团的初始瞬时极性相互作用,然后整个八肽素分子渗透到脂肪酰基中外膜的核心。该机制与特异性针对脂质A的多粘菌素B相反,而八肽似乎同时靶向脂质A和磷脂。此外,八肽的机制似乎并不高度依赖与脂质A的初始互补性静电相互作用,这说明了它们与抗多粘菌素的革兰氏阴性细菌的脂质A结合的能力,该细菌被阳离子部分修饰,这些阳离子部分可静电排斥阳离子多粘菌素分子。提出的发现为八肽蛋白渗透多粘菌抗性革兰氏阴性病原体的外膜提供了新的机制,并突出了它们作为抗病多药耐药性病原体的新抗生素开发的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号