当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
To Photoredox or Not in Neutral Aqueous Solutions for Selected Benzophenone and Anthraquinone Derivatives
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2016-11-16 00:00:00 , DOI: 10.1021/acs.jpclett.6b02403 Xiting Zhang 1, 2 , Jiani Ma 1 , David Lee Phillips 2
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2016-11-16 00:00:00 , DOI: 10.1021/acs.jpclett.6b02403 Xiting Zhang 1, 2 , Jiani Ma 1 , David Lee Phillips 2
Affiliation
|
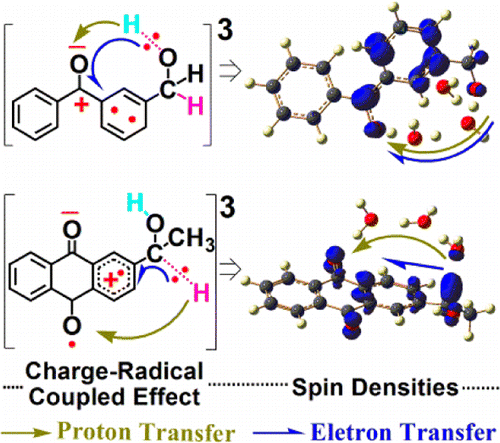
The experimental and theoretical results in neutral aqueous solutions reported here indicate that a proton-coupled electron transfer (PCET) from an alcohol C–H bond to the para-carbonyl is the initial and crucial process for the photoredox reaction of 2-(1-hydroxyethyl)-anthraquinone (HEAQ) to occur while the counterpart 3-(hydroxymethyl)-benzophenone (3-BPOH) compound displays a different PCET from an alcohol O–H bond to the carbonyl as the first step, followed by an intersystem crossing process that does not lead to the analogous photoredox, which is caused by a subtle charge-radical coupled effect between HEAQ and 3-BPOH. This can account for experimental results in the literature that HEAQ can undergo efficient photoredox but 3-BPOH does not under neutral aqueous conditions. These results have implications for the pH-dependent photochemical behavior of aromatic carbonyl compounds in aqueous media.
中文翻译:

选择性苯甲酮和蒽醌衍生物在中性水溶液中的光氧化还原与否
此处报道的在中性水溶液中的实验和理论结果表明,从醇C–H键到对位的质子偶联电子转移(PCET)-羰基是2-(1-羟乙基)-蒽醌(HEAQ)发生光氧化还原反应的初始且至关重要的过程,而对应的3-(羟甲基)-二苯甲酮(3-BPOH)化合物显示出与醇不同的PCET第一步,OH键与羰基键合,然后进行系统间穿越过程,该过程不会导致类似的光氧化还原,这是由HEAQ和3-BPOH之间的微妙的电荷-自由基耦合作用引起的。这可以解释文献中的实验结果,即HEAQ可以进行有效的光氧化还原,而3-BPOH在中性水溶液条件下则不能。这些结果暗示了芳族羰基化合物在水性介质中的pH依赖性光化学行为。
更新日期:2016-11-16
中文翻译:

选择性苯甲酮和蒽醌衍生物在中性水溶液中的光氧化还原与否
此处报道的在中性水溶液中的实验和理论结果表明,从醇C–H键到对位的质子偶联电子转移(PCET)-羰基是2-(1-羟乙基)-蒽醌(HEAQ)发生光氧化还原反应的初始且至关重要的过程,而对应的3-(羟甲基)-二苯甲酮(3-BPOH)化合物显示出与醇不同的PCET第一步,OH键与羰基键合,然后进行系统间穿越过程,该过程不会导致类似的光氧化还原,这是由HEAQ和3-BPOH之间的微妙的电荷-自由基耦合作用引起的。这可以解释文献中的实验结果,即HEAQ可以进行有效的光氧化还原,而3-BPOH在中性水溶液条件下则不能。这些结果暗示了芳族羰基化合物在水性介质中的pH依赖性光化学行为。











































 京公网安备 11010802027423号
京公网安备 11010802027423号