当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Insights into Seeding Mechanisms of hIAPP Fibril Formation
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-05-09 , DOI: 10.1021/jacs.3c14233 Saba Suladze 1, 2 , Christian Sustay Martinez 3 , Diana C. Rodriguez Camargo 1, 2 , Jonas Engler 1, 2 , Natalia Rodina 1, 2 , Riddhiman Sarkar 1, 2 , Martin Zacharias 3 , Bernd Reif 1, 2
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-05-09 , DOI: 10.1021/jacs.3c14233 Saba Suladze 1, 2 , Christian Sustay Martinez 3 , Diana C. Rodriguez Camargo 1, 2 , Jonas Engler 1, 2 , Natalia Rodina 1, 2 , Riddhiman Sarkar 1, 2 , Martin Zacharias 3 , Bernd Reif 1, 2
Affiliation
|
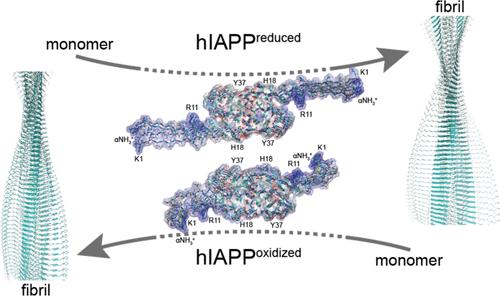
The deposition of islet amyloid polypeptide (hIAPP) fibrils is a hallmark of β-cell death in type II diabetes. In this study, we employ state-of-the-art MAS solid-state spectroscopy to investigate the previously elusive N-terminal region of hIAPP fibrils, uncovering both rigidity and heterogeneity. Comparative analysis between wild-type hIAPP and a disulfide-deficient variant (hIAPPC2S,C7S) unveils shared fibril core structures yet strikingly distinct dynamics in the N-terminus. Specifically, the variant fibrils exhibit extended β-strand conformations, facilitating surface nucleation. Moreover, our findings illuminate the pivotal roles of specific residues in modulating secondary nucleation rates. These results deepen our understanding of hIAPP fibril assembly and provide critical insights into the molecular mechanisms underpinning type II diabetes, holding promise for future therapeutic strategies.
中文翻译:

hIAPP 原纤维形成的播种机制的结构见解
胰岛淀粉样多肽 (hIAPP) 原纤维的沉积是 II 型糖尿病中 β 细胞死亡的标志。在这项研究中,我们采用最先进的 MAS 固态光谱来研究以前难以捉摸的 hIAPP 原纤维的 N 端区域,揭示刚性和异质性。野生型 hIAPP 和二硫键缺陷变体 (hIAPP C2S,C7S ) 之间的比较分析揭示了共享的原纤维核心结构,但 N 末端的动态却截然不同。具体来说,变体原纤维表现出延伸的β链构象,促进表面成核。此外,我们的研究结果阐明了特定残基在调节二次成核速率中的关键作用。这些结果加深了我们对 hIAPP 原纤维组装的理解,并为 II 型糖尿病的分子机制提供了重要的见解,为未来的治疗策略带来了希望。
更新日期:2024-05-10
中文翻译:

hIAPP 原纤维形成的播种机制的结构见解
胰岛淀粉样多肽 (hIAPP) 原纤维的沉积是 II 型糖尿病中 β 细胞死亡的标志。在这项研究中,我们采用最先进的 MAS 固态光谱来研究以前难以捉摸的 hIAPP 原纤维的 N 端区域,揭示刚性和异质性。野生型 hIAPP 和二硫键缺陷变体 (hIAPP C2S,C7S ) 之间的比较分析揭示了共享的原纤维核心结构,但 N 末端的动态却截然不同。具体来说,变体原纤维表现出延伸的β链构象,促进表面成核。此外,我们的研究结果阐明了特定残基在调节二次成核速率中的关键作用。这些结果加深了我们对 hIAPP 原纤维组装的理解,并为 II 型糖尿病的分子机制提供了重要的见解,为未来的治疗策略带来了希望。






























 京公网安备 11010802027423号
京公网安备 11010802027423号