当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Predicting the Soret Coefficient of Molecular Binary Mixtures
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2024-05-08 , DOI: 10.1021/acs.iecr.4c00818 Konstantin I. Morozov 1
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2024-05-08 , DOI: 10.1021/acs.iecr.4c00818 Konstantin I. Morozov 1
Affiliation
|
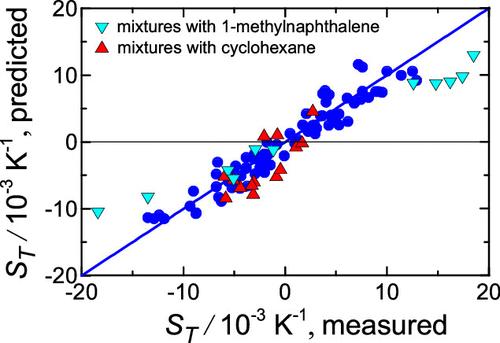
Thermodiffusion of binary mixtures in an inhomogeneous temperature field is due to scattering phenomena and the effects of interparticle interactions. Accordingly, when describing the Soret coefficient, it is customary to distinguish between the kinetic and the chemical contributions. Predicting thermodiffusion even in simple binary mixtures is challenging because of numerous factors that influence these contributions. Here, we present a comprehensive theoretical study of both contributions and make predictions of the Soret coefficient for 141 equimolar binary mixtures composed of 24 nonassociating and non- or weakly polar simple liquids. The chemical contribution is an equilibrium property related to the gradient of a partial pressure of one of the mixture components. It is successfully described within the framework of the perturbed-chain statistical associating fluid theory, characterizing the pure liquids by only three physically significant parameters: the segment number, the hard-core segment diameter, and the segment–segment interaction parameter. The nonequilibrium kinetic contribution is approximated by the relation associated with hydrodynamic fluctuations. The sum of the chemical and kinetic contributions determines the Soret coefficient of the mixture. Both contributions are found for the case of dilute mixtures. The equimolar value of the Soret coefficient is calculated as the arithmetic mean of its limiting values. The predicted values of the Soret coefficient are compared with the experimental data available for 113 mixtures. Overall, the theoretical and experimental results are in good agreement with each other. For 70 mixtures, the difference of data does not exceed 0.002 K–1. The best agreement between the data occurs for the alkanes. The maximum deviations of the results are observed for mixtures with 1-methylnaphthalene, bromonaphthalene, and cyclohexane. We relate these deviations to the plate-like shape of their molecules, which is not taken into account when deriving the kinetic contribution.
中文翻译:

预测分子二元混合物的 Soret 系数
二元混合物在不均匀温度场中的热扩散是由于散射现象和颗粒间相互作用的影响。因此,在描述索雷特系数时,通常区分动力学贡献和化学贡献。即使在简单的二元混合物中预测热扩散也具有挑战性,因为影响这些贡献的因素有很多。在这里,我们对这两种贡献进行了全面的理论研究,并对由 24 种非缔合和非或弱极性简单液体组成的 141 种等摩尔二元混合物的 Soret 系数进行了预测。化学贡献是与混合物组分之一的分压梯度相关的平衡特性。它在扰动链统计关联流体理论的框架内得到了成功的描述,仅通过三个物理上重要的参数来表征纯液体:段数、核心段直径和段与段相互作用参数。非平衡动力学贡献通过与流体动力学波动相关的关系来近似。化学和动力学贡献的总和决定了混合物的索雷系数。这两种贡献都是在稀混合物的情况下发现的。索雷系数的等摩尔值计算为其极限值的算术平均值。将 Soret 系数的预测值与 113 种混合物的实验数据进行了比较。总体而言,理论和实验结果非常吻合。对于 70 种混合物,数据差异不超过 0.002 K –1 。对于烷烃,数据之间的一致性最好。 对于与 1-甲基萘、溴萘和环己烷的混合物,观察到结果的最大偏差。我们将这些偏差与其分子的板状形状联系起来,在推导动力学贡献时没有考虑到这一点。
更新日期:2024-05-08
中文翻译:

预测分子二元混合物的 Soret 系数
二元混合物在不均匀温度场中的热扩散是由于散射现象和颗粒间相互作用的影响。因此,在描述索雷特系数时,通常区分动力学贡献和化学贡献。即使在简单的二元混合物中预测热扩散也具有挑战性,因为影响这些贡献的因素有很多。在这里,我们对这两种贡献进行了全面的理论研究,并对由 24 种非缔合和非或弱极性简单液体组成的 141 种等摩尔二元混合物的 Soret 系数进行了预测。化学贡献是与混合物组分之一的分压梯度相关的平衡特性。它在扰动链统计关联流体理论的框架内得到了成功的描述,仅通过三个物理上重要的参数来表征纯液体:段数、核心段直径和段与段相互作用参数。非平衡动力学贡献通过与流体动力学波动相关的关系来近似。化学和动力学贡献的总和决定了混合物的索雷系数。这两种贡献都是在稀混合物的情况下发现的。索雷系数的等摩尔值计算为其极限值的算术平均值。将 Soret 系数的预测值与 113 种混合物的实验数据进行了比较。总体而言,理论和实验结果非常吻合。对于 70 种混合物,数据差异不超过 0.002 K –1 。对于烷烃,数据之间的一致性最好。 对于与 1-甲基萘、溴萘和环己烷的混合物,观察到结果的最大偏差。我们将这些偏差与其分子的板状形状联系起来,在推导动力学贡献时没有考虑到这一点。































 京公网安备 11010802027423号
京公网安备 11010802027423号