当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reducing Cathodic Drift during Isoelectric Focusing Using Microscale Immobilized pH Gradient Gels
Analytical Chemistry ( IF 7.4 ) Pub Date : 2024-05-08 , DOI: 10.1021/acs.analchem.4c00788 Gabriela Lomeli 1, 2 , Amy E. Herr 1, 2, 3
Analytical Chemistry ( IF 7.4 ) Pub Date : 2024-05-08 , DOI: 10.1021/acs.analchem.4c00788 Gabriela Lomeli 1, 2 , Amy E. Herr 1, 2, 3
Affiliation
|
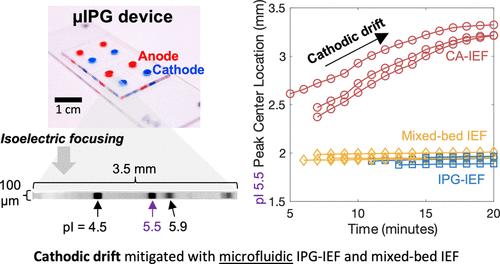
Microfluidic analytical tools play an important role in miniaturizing targeted proteomic assays for improved detection sensitivity, throughput, and automation. Microfluidic isoelectric focusing (IEF) can resolve proteoforms in lysate from low-to-single cell numbers. However, IEF assays often use carrier ampholytes (CAs) to establish a pH gradient for protein separation, presenting limitations like pH instability in the form of cathodic drift (migration of focused proteins toward the cathode). Immobilized pH gradient (IPG) gels reduce cathodic drift by covalently immobilizing the pH buffering components to a matrix. To our knowledge, efforts to implement IPG gels at the microscale have been limited to glass microdevices. To adapt IEF using IPGs to widely used microfluidic device materials, we introduce a polydimethylsiloxane (PDMS)-based microfluidic device and compare the microscale pH gradient stability of IEF established with IPGs, CAs, and a hybrid formulation of IPG gels and CAs (mixed-bed IEF). The PDMS-based IPG microfluidic device (μIPG) resolved analytes differing by 0.1 isoelectric point within a 3.5 mm separation lane over a 20 min focusing duration. During the 20 min duration, we observed markedly different cathodic drift velocities among the three formulations: 60.1 μm/min in CA-IEF, 2.5 μm/min in IPG-IEF (∼24-fold reduction versus CA-IEF), and 1.4 μm/min in mixed-bed IEF (∼43-fold reduction versus CA-IEF). Lastly, mixed-bed IEF in a PDMS device resolved green fluorescent protein (GFP) proteoforms from GFP-expressing human breast cancer cell lysate, thus establishing stability in lysate from complex biospecimens. μIPG is a promising and stable technique for studying proteoforms from small volumes.
中文翻译:

使用微型固定 pH 梯度凝胶减少等电聚焦过程中的阴极漂移
微流体分析工具在小型化靶向蛋白质组检测以提高检测灵敏度、通量和自动化方面发挥着重要作用。微流控等电聚焦 (IEF) 可以解析裂解液中低至单细胞数量的蛋白质形式。然而,IEF 测定通常使用载体两性电解质 (CA) 来建立用于蛋白质分离的 pH 梯度,从而存在一些局限性,例如以阴极漂移(聚焦的蛋白质向阴极迁移)形式出现的 pH 不稳定。固定化 pH 梯度 (IPG) 凝胶通过将 pH 缓冲成分共价固定到基质上来减少阴极漂移。据我们所知,在微型尺度上实施 IPG 凝胶的努力仅限于玻璃微型器件。为了使使用 IPG 的 IEF 适应广泛使用的微流体装置材料,我们引入了一种基于聚二甲基硅氧烷 (PDMS) 的微流体装置,并比较了用 IPG、CA 以及 IPG 凝胶和 CA 的混合配方建立的 IEF 的微尺度 pH 梯度稳定性。床 IEF)。基于 PDMS 的 IPG 微流控装置 (μIPG) 在 20 分钟的聚焦时间内解析了 3.5 毫米分离泳道内等电点相差 0.1 的分析物。在 20 分钟的持续时间内,我们观察到三种配方之间的阴极漂移速度明显不同:CA-IEF 中为 60.1 μm/min,IPG-IEF 中为 2.5 μm/min(与 CA-IEF 相比减少约 24 倍),以及 1.4 μm/min /min 在混合床 IEF 中(与 CA-IEF 相比减少约 43 倍)。最后,PDMS 装置中的混床 IEF 解析了表达 GFP 的人乳腺癌细胞裂解物中的绿色荧光蛋白 (GFP) 蛋白质形式,从而建立了复杂生物样本裂解物的稳定性。 μIPG 是一种用于研究小体积蛋白质形式的有前途且稳定的技术。
更新日期:2024-05-08
中文翻译:

使用微型固定 pH 梯度凝胶减少等电聚焦过程中的阴极漂移
微流体分析工具在小型化靶向蛋白质组检测以提高检测灵敏度、通量和自动化方面发挥着重要作用。微流控等电聚焦 (IEF) 可以解析裂解液中低至单细胞数量的蛋白质形式。然而,IEF 测定通常使用载体两性电解质 (CA) 来建立用于蛋白质分离的 pH 梯度,从而存在一些局限性,例如以阴极漂移(聚焦的蛋白质向阴极迁移)形式出现的 pH 不稳定。固定化 pH 梯度 (IPG) 凝胶通过将 pH 缓冲成分共价固定到基质上来减少阴极漂移。据我们所知,在微型尺度上实施 IPG 凝胶的努力仅限于玻璃微型器件。为了使使用 IPG 的 IEF 适应广泛使用的微流体装置材料,我们引入了一种基于聚二甲基硅氧烷 (PDMS) 的微流体装置,并比较了用 IPG、CA 以及 IPG 凝胶和 CA 的混合配方建立的 IEF 的微尺度 pH 梯度稳定性。床 IEF)。基于 PDMS 的 IPG 微流控装置 (μIPG) 在 20 分钟的聚焦时间内解析了 3.5 毫米分离泳道内等电点相差 0.1 的分析物。在 20 分钟的持续时间内,我们观察到三种配方之间的阴极漂移速度明显不同:CA-IEF 中为 60.1 μm/min,IPG-IEF 中为 2.5 μm/min(与 CA-IEF 相比减少约 24 倍),以及 1.4 μm/min /min 在混合床 IEF 中(与 CA-IEF 相比减少约 43 倍)。最后,PDMS 装置中的混床 IEF 解析了表达 GFP 的人乳腺癌细胞裂解物中的绿色荧光蛋白 (GFP) 蛋白质形式,从而建立了复杂生物样本裂解物的稳定性。 μIPG 是一种用于研究小体积蛋白质形式的有前途且稳定的技术。






























 京公网安备 11010802027423号
京公网安备 11010802027423号