当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enzymatic and Algebraic Methodology to Determine the Contents of Kunitz and Bowman–Birk Inhibitors and Their Contributions to Total Trypsin or Chymotrypsin Inhibition in Soybeans
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2024-05-08 , DOI: 10.1021/acs.jafc.3c06389 Keshun Liu 1
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2024-05-08 , DOI: 10.1021/acs.jafc.3c06389 Keshun Liu 1
Affiliation
|
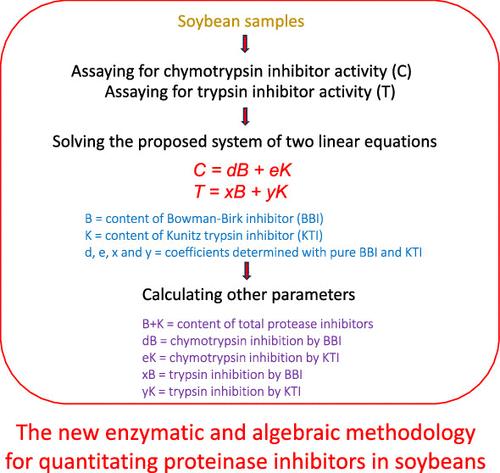
Soybeans are the number one source of plant proteins for food and feed, but the natural presence of protein protease inhibitors (PIs), namely, the Kunitz trypsin inhibitor (KTI) and the Bowman–Birk inhibitor (BBI), exerts antinutritional effects. This communication describes a new methodology for simultaneously quantitating all parameters of PIs in soybeans. It consists of seven steps and featured enzymatically measuring trypsin and chymotrypsin inhibitory activities, respectively, and subsequently determining the contents of reactive KTI and BBI and the contributions of each toward total PI mass and total trypsin or chymotrypsin inhibition by solving a proposed system of linear equations with two variables (C = dB + eK and T = xB + yK). This enzymatic and algebraic (EA) methodology was based on differential inhibitions of KTI and BBI toward trypsin and chymotrypsin and validated by applications to a series of mixtures of purified KTI and BBI, two KTI-null and two conventional soybeans, and by sodium dodecyl sulfate polyacrylamide gel electrophoresis. The EA methodology allowed calculations of PI composition and the contributions of individual inhibitors toward total inhibition with ease. It was first found that although BBI constituted only about 30% of the total PI mass in conventional raw soybeans, it contributed about 80% toward total chymotrypsin inhibitor activity and about 45% toward trypsin inhibitor activity. Therefore, BBI caused more total protease inhibitions than those of KTI. Furthermore, the so-called KTI-null soybean mutants still contained measurable KTI content and thus should be named KTI-low soybeans.
中文翻译:

用酶学和代数方法测定大豆中 Kunitz 和 Bowman-Birk 抑制剂的含量及其对总胰蛋白酶或糜蛋白酶抑制的贡献
大豆是食品和饲料中植物蛋白的第一来源,但天然存在的蛋白质蛋白酶抑制剂 (PI),即库尼兹胰蛋白酶抑制剂 (KTI) 和鲍曼-伯克抑制剂 (BBI),会产生抗营养作用。该通讯描述了一种同时定量大豆中所有 PI 参数的新方法。它由七个步骤组成,分别以酶法测量胰蛋白酶和糜蛋白酶抑制活性,随后通过求解线性方程组确定反应性 KTI 和 BBI 的含量以及它们对总 PI 质量和总胰蛋白酶或糜蛋白酶抑制的贡献有两个变量(C = dB + eK和T = xB + yK)。这种酶代数 (EA) 方法基于 KTI 和 BBI 对胰蛋白酶和糜蛋白酶的差异抑制,并通过应用一系列纯化 KTI 和 BBI、两种 KTI 无效大豆和两种常规大豆以及十二烷基硫酸钠的混合物进行验证聚丙烯酰胺凝胶电泳。 EA 方法可以轻松计算 PI 组成以及单个抑制剂对总抑制的贡献。首次发现,尽管BBI仅占传统生大豆中PI总质量的约30%,但其对胰凝乳蛋白酶抑制剂总活性的贡献率约为80%,对胰蛋白酶抑制剂活性的贡献率约为45%。因此,BBI 比 KTI 引起更多的总蛋白酶抑制。此外,所谓的KTI无效大豆突变体仍然含有可测量的KTI含量,因此应命名为KTI低大豆。
更新日期:2024-05-08
中文翻译:

用酶学和代数方法测定大豆中 Kunitz 和 Bowman-Birk 抑制剂的含量及其对总胰蛋白酶或糜蛋白酶抑制的贡献
大豆是食品和饲料中植物蛋白的第一来源,但天然存在的蛋白质蛋白酶抑制剂 (PI),即库尼兹胰蛋白酶抑制剂 (KTI) 和鲍曼-伯克抑制剂 (BBI),会产生抗营养作用。该通讯描述了一种同时定量大豆中所有 PI 参数的新方法。它由七个步骤组成,分别以酶法测量胰蛋白酶和糜蛋白酶抑制活性,随后通过求解线性方程组确定反应性 KTI 和 BBI 的含量以及它们对总 PI 质量和总胰蛋白酶或糜蛋白酶抑制的贡献有两个变量(C = dB + eK和T = xB + yK)。这种酶代数 (EA) 方法基于 KTI 和 BBI 对胰蛋白酶和糜蛋白酶的差异抑制,并通过应用一系列纯化 KTI 和 BBI、两种 KTI 无效大豆和两种常规大豆以及十二烷基硫酸钠的混合物进行验证聚丙烯酰胺凝胶电泳。 EA 方法可以轻松计算 PI 组成以及单个抑制剂对总抑制的贡献。首次发现,尽管BBI仅占传统生大豆中PI总质量的约30%,但其对胰凝乳蛋白酶抑制剂总活性的贡献率约为80%,对胰蛋白酶抑制剂活性的贡献率约为45%。因此,BBI 比 KTI 引起更多的总蛋白酶抑制。此外,所谓的KTI无效大豆突变体仍然含有可测量的KTI含量,因此应命名为KTI低大豆。






























 京公网安备 11010802027423号
京公网安备 11010802027423号