当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Multiscale Gas–Solid Reaction Dynamics of Hematite Oxygen Carrier in Chemical Looping Combustion from Fluidized Bed Thermogravimetric Analysis
Energy & Fuels ( IF 5.3 ) Pub Date : 2024-05-08 , DOI: 10.1021/acs.energyfuels.4c00853 Tianxu Shen 1 , Laihong Shen 2 , Tao Song 1
Energy & Fuels ( IF 5.3 ) Pub Date : 2024-05-08 , DOI: 10.1021/acs.energyfuels.4c00853 Tianxu Shen 1 , Laihong Shen 2 , Tao Song 1
Affiliation
|
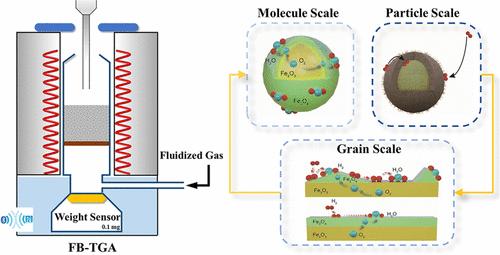
The pursuit of precise and detailed kinetic characteristics continues to drive advancements in both modeling and measurement methodologies. A multiscale kinetic model was developed to analyze the redox behaviors of the hematite oxygen carrier (OC), characterized by a fluidized bed thermogravimetric analyzer (FB-TGA). This multiscale model integrates bulk diffusion of lattice oxygen, discrete growth of product islands, particle-scale gas diffusion, and interphase gas exchange, offering a comprehensive insight into the evolution of reaction rate and mass transfer. The investigation revealed that the surface diffusion rates of gas molecules are the crucial determinant in the Fe2O3 reactivity, with rapid surface diffusion delaying the formation of an enclosed product layer. The exceptional diffusion conditions and precise weight measurement capability of FB-TGA yielded faster reaction rate constants than conventional methods, facilitating transformative insights into rate-limiting factors. External diffusion and bulk lattice oxygen diffusion were identified as rapid and insufficient in imposing limitations on the overall reaction. Conversely, intraparticle diffusion emerged as the primary rate-limiting step in kinetic experiments even involving 0.1–0.15 mm hematite OC particles, especially during the initial oxidation of Fe3O4, where the mass transfer efficiency dropped below 85%. In actual chemical looping combustion environments utilizing 0.3–0.45 mm hematite OC particles, the resistance of intraparticle diffusion significantly intensified, inhibiting gas–solid reaction rates to an extent comparable to interphase mass transfer. Based on the insights derived from the multiscale model analysis, strategies for optimizing OC particle structure and reactor design have been proposed to enhance gas–solid reactions.
中文翻译:

流化床热重分析化学循环燃烧中赤铁矿载氧体的多尺度气固反应动力学
对精确和详细的动力学特性的追求继续推动建模和测量方法的进步。开发了多尺度动力学模型来分析赤铁矿氧载体(OC)的氧化还原行为,并通过流化床热重分析仪(FB-TGA)进行表征。该多尺度模型集成了晶格氧的本体扩散、产物岛的离散生长、颗粒尺度气体扩散和相间气体交换,提供了对反应速率和传质演化的全面洞察。研究表明,气体分子的表面扩散速率是 Fe 2 O 3反应性的关键决定因素,快速的表面扩散延迟了封闭产物层的形成。 FB-TGA 卓越的扩散条件和精确的重量测量能力比传统方法产生更快的反应速率常数,有助于对速率限制因素进行变革性的了解。外部扩散和体晶格氧扩散被认为是快速的并且不足以对整个反应施加限制。相反,颗粒内扩散成为动力学实验中的主要限速步骤,甚至涉及 0.1–0.15 mm 赤铁矿 OC 颗粒,特别是在 Fe 3 O 4的初始氧化过程中,传质效率降至 85% 以下。在使用 0.3-0.45 mm 赤铁矿 OC 颗粒的实际化学链燃烧环境中,颗粒内扩散的阻力显着增强,抑制气固反应速率的程度与相间传质相当。基于多尺度模型分析得出的见解,提出了优化 OC 颗粒结构和反应器设计的策略,以增强气固反应。
更新日期:2024-05-08
中文翻译:

流化床热重分析化学循环燃烧中赤铁矿载氧体的多尺度气固反应动力学
对精确和详细的动力学特性的追求继续推动建模和测量方法的进步。开发了多尺度动力学模型来分析赤铁矿氧载体(OC)的氧化还原行为,并通过流化床热重分析仪(FB-TGA)进行表征。该多尺度模型集成了晶格氧的本体扩散、产物岛的离散生长、颗粒尺度气体扩散和相间气体交换,提供了对反应速率和传质演化的全面洞察。研究表明,气体分子的表面扩散速率是 Fe 2 O 3反应性的关键决定因素,快速的表面扩散延迟了封闭产物层的形成。 FB-TGA 卓越的扩散条件和精确的重量测量能力比传统方法产生更快的反应速率常数,有助于对速率限制因素进行变革性的了解。外部扩散和体晶格氧扩散被认为是快速的并且不足以对整个反应施加限制。相反,颗粒内扩散成为动力学实验中的主要限速步骤,甚至涉及 0.1–0.15 mm 赤铁矿 OC 颗粒,特别是在 Fe 3 O 4的初始氧化过程中,传质效率降至 85% 以下。在使用 0.3-0.45 mm 赤铁矿 OC 颗粒的实际化学链燃烧环境中,颗粒内扩散的阻力显着增强,抑制气固反应速率的程度与相间传质相当。基于多尺度模型分析得出的见解,提出了优化 OC 颗粒结构和反应器设计的策略,以增强气固反应。
































 京公网安备 11010802027423号
京公网安备 11010802027423号