Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Antibiotic‐Induced Gut Microbiota Dysbiosis Modulates Host Transcriptome and m6A Epitranscriptome via Bile Acid Metabolism
Advanced Science ( IF 15.1 ) Pub Date : 2024-05-07 , DOI: 10.1002/advs.202307981 Meng Yang 1 , Xiaoqi Zheng 1, 2 , Jiajun Fan 1 , Wei Cheng 3 , Tong‐Meng Yan 4 , Yushan Lai 1 , Nianping Zhang 1 , Yi Lu 1, 2 , Jiali Qi 1 , Zhengyi Huo 1 , Zihe Xu 1, 2 , Jia Huang 1 , Yuting Jiao 1 , Biaodi Liu 5 , Rui Pang 6 , Xiang Zhong 7 , Shi Huang 8 , Guan‐Zheng Luo 5 , Gina Lee 9 , Christian Jobin 10 , A. Murat Eren 11, 12 , Eugene B Chang 13 , Hong Wei 3 , Tao Pan 14 , Xiaoyun Wang 1, 2, 15
Advanced Science ( IF 15.1 ) Pub Date : 2024-05-07 , DOI: 10.1002/advs.202307981 Meng Yang 1 , Xiaoqi Zheng 1, 2 , Jiajun Fan 1 , Wei Cheng 3 , Tong‐Meng Yan 4 , Yushan Lai 1 , Nianping Zhang 1 , Yi Lu 1, 2 , Jiali Qi 1 , Zhengyi Huo 1 , Zihe Xu 1, 2 , Jia Huang 1 , Yuting Jiao 1 , Biaodi Liu 5 , Rui Pang 6 , Xiang Zhong 7 , Shi Huang 8 , Guan‐Zheng Luo 5 , Gina Lee 9 , Christian Jobin 10 , A. Murat Eren 11, 12 , Eugene B Chang 13 , Hong Wei 3 , Tao Pan 14 , Xiaoyun Wang 1, 2, 15
Affiliation
|
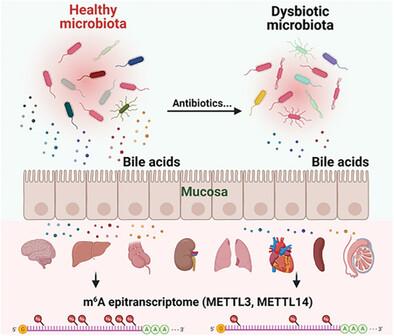
Gut microbiota can influence host gene expression and physiology through metabolites. Besides, the presence or absence of gut microbiome can reprogram host transcriptome and epitranscriptome as represented by N 6 ‐methyladenosine (m6 A), the most abundant mammalian mRNA modification. However, which and how gut microbiota‐derived metabolites reprogram host transcriptome and m6 A epitranscriptome remain poorly understood. Here, investigation is conducted into how gut microbiota‐derived metabolites impact host transcriptome and m6 A epitranscriptome using multiple mouse models and multi‐omics approaches. Various antibiotics‐induced dysbiotic mice are established, followed by fecal microbiota transplantation (FMT) into germ‐free mice, and the results show that bile acid metabolism is significantly altered along with the abundance change in bile acid‐producing microbiota. Unbalanced gut microbiota and bile acids drastically change the host transcriptome and the m6 A epitranscriptome in multiple tissues. Mechanistically, the expression of m6 A writer proteins is regulated in animals treated with antibiotics and in cultured cells treated with bile acids, indicating a direct link between bile acid metabolism and m6 A biology. Collectively, these results demonstrate that antibiotic‐induced gut dysbiosis regulates the landscape of host transcriptome and m6 A epitranscriptome via bile acid metabolism pathway. This work provides novel insights into the interplay between microbial metabolites and host gene expression.
中文翻译:

抗生素诱导的肠道菌群失调通过胆汁酸代谢调节宿主转录组和 m6A 表观转录组
肠道微生物群可以通过代谢物影响宿主基因表达和生理机能。此外,肠道微生物组的存在或不存在可以重新编程宿主转录组和表观转录组,如下所示氮 6 ‐甲基腺苷(m6 A)、最丰富的哺乳动物mRNA修饰。然而,哪些肠道微生物衍生的代谢物以及如何重新编程宿主转录组和m6 表观转录组仍然知之甚少。在这里,研究了肠道微生物群衍生的代谢物如何影响宿主转录组和m6 使用多种小鼠模型和多组学方法的表观转录组。建立了各种抗生素诱导的生态失调小鼠,然后将粪便微生物群移植(FMT)到无菌小鼠中,结果表明,随着产胆汁酸微生物群丰度的变化,胆汁酸代谢显着改变。不平衡的肠道微生物群和胆汁酸极大地改变了宿主转录组和m6 多个组织中的表观转录组。机械地,m 的表达式6 在用抗生素处理的动物和用胆汁酸处理的培养细胞中,作家蛋白受到调节,这表明胆汁酸代谢与 m 之间存在直接联系。6 生物学。总的来说,这些结果表明抗生素引起的肠道菌群失调调节宿主转录组和m的景观6 通过胆汁酸代谢途径的表观转录组。这项工作为微生物代谢物与宿主基因表达之间的相互作用提供了新的见解。
更新日期:2024-05-07
中文翻译:

抗生素诱导的肠道菌群失调通过胆汁酸代谢调节宿主转录组和 m6A 表观转录组
肠道微生物群可以通过代谢物影响宿主基因表达和生理机能。此外,肠道微生物组的存在或不存在可以重新编程宿主转录组和表观转录组,如下所示






























 京公网安备 11010802027423号
京公网安备 11010802027423号