当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
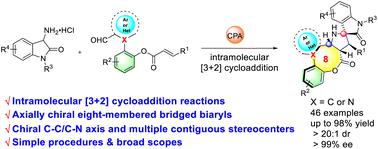
Organocatalytic diastereo- and atropo-selective construction of eight-membered bridged (hetero)biaryls via asymmetric intramolecular [3 + 2] cycloaddition
Chemical Science ( IF 8.4 ) Pub Date : 2024-05-07 , DOI: 10.1039/d4sc01892c Yue Wang 1 , Yue Huang 1 , Xiaoze Bao 2 , Xingfu Wei 1 , Shiqiang Wei 1 , Jingping Qu 1 , Baomin Wang 1
Chemical Science ( IF 8.4 ) Pub Date : 2024-05-07 , DOI: 10.1039/d4sc01892c Yue Wang 1 , Yue Huang 1 , Xiaoze Bao 2 , Xingfu Wei 1 , Shiqiang Wei 1 , Jingping Qu 1 , Baomin Wang 1
Affiliation
|
An unprecedented and straightforward route for the asymmetric construction of privileged atroposelective bridged (hetero)biaryl eight-membered scaffolds has been accomplished through chiral phosphoric acid catalyzed asymmetric intramolecular [3 + 2] cycloaddition of innovative (hetero)biaryl aldehydes with 3-aminooxindole hydrochlorides. A class of eight-membered bridged (hetero)biaryl lactones fused to spiro[pyrrolidine-oxindole] derivatives, possessing both chiral C–C/C–N axes and multiple contiguous stereocenters, were obtained in good yields with excellent enantioselectivities and diastereoselectivities in one step through this direct strategy. In addition, the good scalability and derivatization of the title compounds demonstrated their synthetic utility.
中文翻译:

通过不对称分子内[3 + 2]环加成有机催化非对映和阿托选择性构建八元桥联(杂)联芳基
通过手性磷酸催化创新型(杂)联芳醛与 3-氨基羟吲哚盐酸盐的不对称分子内 [3 + 2] 环加成,实现了一种前所未有的直接途径,用于不对称构建优先的间质选择性桥联(杂)联芳基八元支架。一类与螺[吡咯烷-羟吲哚]衍生物稠合的八元桥联(杂)联芳基内酯,具有手性C-C/C-N轴和多个连续的立构中心,以良好的产率获得,具有优异的对映选择性和非对映选择性。逐步实施这一直接策略。此外,标题化合物良好的可扩展性和衍生性证明了它们的合成实用性。
更新日期:2024-05-07
中文翻译:

通过不对称分子内[3 + 2]环加成有机催化非对映和阿托选择性构建八元桥联(杂)联芳基
通过手性磷酸催化创新型(杂)联芳醛与 3-氨基羟吲哚盐酸盐的不对称分子内 [3 + 2] 环加成,实现了一种前所未有的直接途径,用于不对称构建优先的间质选择性桥联(杂)联芳基八元支架。一类与螺[吡咯烷-羟吲哚]衍生物稠合的八元桥联(杂)联芳基内酯,具有手性C-C/C-N轴和多个连续的立构中心,以良好的产率获得,具有优异的对映选择性和非对映选择性。逐步实施这一直接策略。此外,标题化合物良好的可扩展性和衍生性证明了它们的合成实用性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号