当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rationalizing Counterion Selection for the Development of Lipophilic Salts: A Case Study with Venetoclax
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2024-05-04 , DOI: 10.1021/acs.molpharmaceut.4c00106 Callum D. Ryan 1, 2 , Brendan T. Griffin 1, 2 , Joseph P. O’Shea 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2024-05-04 , DOI: 10.1021/acs.molpharmaceut.4c00106 Callum D. Ryan 1, 2 , Brendan T. Griffin 1, 2 , Joseph P. O’Shea 1
Affiliation
|
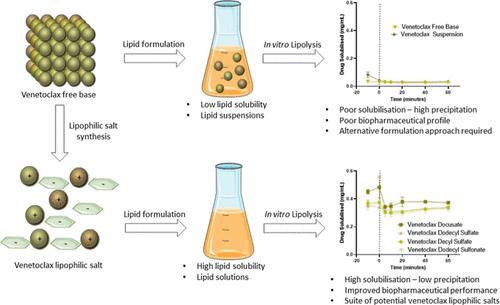
The use of lipid-based formulations (LBFs) can be hindered by low dose loading due to solubility limitations of candidate drugs in lipid vehicles. Formation of lipophilic salts through pairing these drugs with a lipophilic counterion has been demonstrated as a potential means to enhance dose loading in LBFs. This study investigated the screening of appropriate counterions to form lipophilic salts of the BCS class IV drug venetoclax. The physical properties, lipid solubility, and in vitro performance of the salts were analyzed. This study illustrated the versatility of alkyl sulfates and sulfonates as suitable counterions in lipophilic salt synthesis with up to ∼9-fold higher solubility in medium- and long-chain LBFs when compared to that of the free base form of venetoclax. All salts formulated as LBFs displayed superior in vitro performance when compared to the free base form of the drug due to the higher initial drug loadings in LBFs and increased affinity for colloidal species. Further, in vitro studies confirmed that venetoclax lipophilic salt forms using alkyl chain counterions demonstrated comparable in vitro performance to venetoclax docusate, thus reducing the potential for laxative effects related to docusate administration. High levels of the initial dose loading of venetoclax lipophilic salts were retained in a molecularly dispersed state during dispersion and digestion of the formulation, while also demonstrating increased levels of saturation in biorelevant media. The findings of this study suggest that alkyl chain sulfates and sulfonates can act as a suitable alternative counterion to docusate, facilitating the selection of counterions that can unlock the potential to formulate venetoclax as an LBF.
中文翻译:

合理选择抗衡离子以开发亲脂盐:以 Venetoclax 为例
由于候选药物在脂质载体中的溶解度限制,低剂量负荷可能会阻碍脂质制剂(LBF)的使用。通过将这些药物与亲脂性抗衡离子配对形成亲脂性盐已被证明是增强 LBF 剂量负荷的潜在方法。本研究调查了筛选合适的抗衡离子以形成 BCS IV 类药物 Venetoclax 的亲脂盐。分析了盐的物理性质、脂溶性和体外性能。这项研究说明了烷基硫酸盐和磺酸盐作为亲脂盐合成中合适的抗衡离子的多功能性,与游离碱形式的 Venetoclax 相比,在中链和长链 LBF 中的溶解度高出约 9 倍。与药物的游离碱形式相比,所有以 LBF 形式配制的盐都表现出优异的体外性能,因为 LBF 中的初始载药量较高,并且对胶体物质的亲和力增加。此外,体外研究证实,使用烷基链抗衡离子的维奈托克亲脂盐形式表现出与维奈托克多库酯相当的体外性能,从而降低了与多库酯给药相关的通便作用的可能性。在制剂的分散和消化过程中,维奈托克亲脂盐的高水平初始剂量负载保持在分子分散状态,同时也证明了生物相关介质中饱和度的增加。本研究的结果表明,烷基链硫酸盐和磺酸盐可以作为多库酯的合适替代抗衡离子,促进抗衡离子的选择,从而释放将 Venetoclax 配制为 LBF 的潜力。
更新日期:2024-05-04
中文翻译:

合理选择抗衡离子以开发亲脂盐:以 Venetoclax 为例
由于候选药物在脂质载体中的溶解度限制,低剂量负荷可能会阻碍脂质制剂(LBF)的使用。通过将这些药物与亲脂性抗衡离子配对形成亲脂性盐已被证明是增强 LBF 剂量负荷的潜在方法。本研究调查了筛选合适的抗衡离子以形成 BCS IV 类药物 Venetoclax 的亲脂盐。分析了盐的物理性质、脂溶性和体外性能。这项研究说明了烷基硫酸盐和磺酸盐作为亲脂盐合成中合适的抗衡离子的多功能性,与游离碱形式的 Venetoclax 相比,在中链和长链 LBF 中的溶解度高出约 9 倍。与药物的游离碱形式相比,所有以 LBF 形式配制的盐都表现出优异的体外性能,因为 LBF 中的初始载药量较高,并且对胶体物质的亲和力增加。此外,体外研究证实,使用烷基链抗衡离子的维奈托克亲脂盐形式表现出与维奈托克多库酯相当的体外性能,从而降低了与多库酯给药相关的通便作用的可能性。在制剂的分散和消化过程中,维奈托克亲脂盐的高水平初始剂量负载保持在分子分散状态,同时也证明了生物相关介质中饱和度的增加。本研究的结果表明,烷基链硫酸盐和磺酸盐可以作为多库酯的合适替代抗衡离子,促进抗衡离子的选择,从而释放将 Venetoclax 配制为 LBF 的潜力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号