当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Formation of Volatile Pyrazinones in the Asparagine Maillard Reaction Systems and Novel Pyrazinone Formation Pathways in the Amidated-Alanine Maillard Reaction Systems
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2024-05-02 , DOI: 10.1021/acs.jafc.4c02079 Yue Luo 1 , Run Li 1 , Siyue Zhu 1 , Jie Peng 1 , Qingrong Huang 1 , Tiantian Zhao 2 , Chi-Tang Ho 1
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2024-05-02 , DOI: 10.1021/acs.jafc.4c02079 Yue Luo 1 , Run Li 1 , Siyue Zhu 1 , Jie Peng 1 , Qingrong Huang 1 , Tiantian Zhao 2 , Chi-Tang Ho 1
Affiliation
|
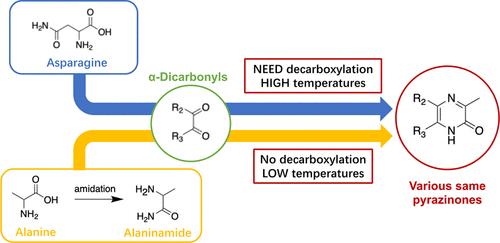
Maillard reaction (MR) plays a pivotal role in the food flavor industry, including a cascade of reactions starting with the reaction between amino compounds and reducing sugars, and thus provides various colors and flavors. A new group of volatile compounds called pyrazinones found in MR are now getting more attention. In this study, eight volatile pyrazinones were found in the asparagine MR systems, in which 3,5-dimethyl- and 3,6-dimethyl-2(1H)-pyrazinones were reported for the first time. The major formation pathways were the reactions between asparagine and α-dicarbonyls, with decarboxylation as a critical step. Besides, novel alternative pathways involving alanine amidation and successive reactions with α-dicarbonyls were explored and successfully formed eight pyrazinones. The major differences between alanine-amidated pathways and decarboxylation pathways are the amidation step and absence of the decarboxylation step. For the alanine-amidated pathways, the higher the temperature, the better the amidation effect. The optimal amidation temperature was 200 °C in this study. The reaction between the alanine amide and α-dicarbonyls after amidation can happen at low temperatures, such as 35 and 50 °C, proposing the possibility of pyrazinone formation in real food systems. Further investigations should be conducted to investigate volatile pyrazinones in various food systems as well as the biological effects and kinetic formation differences of the volatile pyrazinones.
中文翻译:

天冬酰胺美拉德反应系统中挥发性吡嗪酮的形成以及酰胺化-丙氨酸美拉德反应系统中新型吡嗪酮形成途径
美拉德反应(MR)在食品香料行业中发挥着举足轻重的作用,包括从氨基化合物和还原糖之间的反应开始的一系列反应,从而提供各种颜色和风味。在 MR 中发现的一组新的挥发性化合物,称为吡嗪酮,现在正受到更多关注。本研究在天冬酰胺MR系统中发现了8种挥发性吡嗪酮类化合物,其中3,5-二甲基-和3,6-二甲基-2(1H ) -吡嗪酮类化合物为首次报道。主要的形成途径是天冬酰胺和α-二羰基之间的反应,其中脱羧是关键步骤。此外,还探索了涉及丙氨酸酰胺化和与α-二羰基连续反应的新替代途径,并成功形成了八种吡嗪酮。丙氨酸酰胺化途径和脱羧途径之间的主要区别是酰胺化步骤和缺乏脱羧步骤。对于丙氨酸酰胺化途径,温度越高,酰胺化效果越好。本研究中最佳酰胺化温度为200℃。酰胺化后丙氨酸酰胺和α-二羰基之间的反应可以在低温下发生,例如35和50°C,这表明在实际食品系统中形成吡嗪酮的可能性。应进一步研究不同食品体系中的挥发性吡嗪酮类化合物以及挥发性吡嗪酮类化合物的生物效应和动力学形成差异。
更新日期:2024-05-02
中文翻译:

天冬酰胺美拉德反应系统中挥发性吡嗪酮的形成以及酰胺化-丙氨酸美拉德反应系统中新型吡嗪酮形成途径
美拉德反应(MR)在食品香料行业中发挥着举足轻重的作用,包括从氨基化合物和还原糖之间的反应开始的一系列反应,从而提供各种颜色和风味。在 MR 中发现的一组新的挥发性化合物,称为吡嗪酮,现在正受到更多关注。本研究在天冬酰胺MR系统中发现了8种挥发性吡嗪酮类化合物,其中3,5-二甲基-和3,6-二甲基-2(1H ) -吡嗪酮类化合物为首次报道。主要的形成途径是天冬酰胺和α-二羰基之间的反应,其中脱羧是关键步骤。此外,还探索了涉及丙氨酸酰胺化和与α-二羰基连续反应的新替代途径,并成功形成了八种吡嗪酮。丙氨酸酰胺化途径和脱羧途径之间的主要区别是酰胺化步骤和缺乏脱羧步骤。对于丙氨酸酰胺化途径,温度越高,酰胺化效果越好。本研究中最佳酰胺化温度为200℃。酰胺化后丙氨酸酰胺和α-二羰基之间的反应可以在低温下发生,例如35和50°C,这表明在实际食品系统中形成吡嗪酮的可能性。应进一步研究不同食品体系中的挥发性吡嗪酮类化合物以及挥发性吡嗪酮类化合物的生物效应和动力学形成差异。






























 京公网安备 11010802027423号
京公网安备 11010802027423号