当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
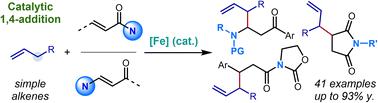
A C–H functionalization approach to diverse nitrogenous scaffolds through conjugate addition of catalytic allyliron nucleophiles
Chemical Science ( IF 8.4 ) Pub Date : 2024-05-01 , DOI: 10.1039/d4sc00655k Sarah G. Scrivener 1 , Yi-Ming Wang 1
Chemical Science ( IF 8.4 ) Pub Date : 2024-05-01 , DOI: 10.1039/d4sc00655k Sarah G. Scrivener 1 , Yi-Ming Wang 1
Affiliation
|
Cyclopentadienyliron(II) dicarbonyl complexes capable of coordinating to and enhancing the acidity of a range of unsaturated substrates have emerged as a new class of base-metal derived catalysts for C–H functionalization. In this manuscript, the iron-catalyzed C–H functionalization of allylic C(sp3)–H bonds using nitrogen containing α,β-unsaturated carbonyl compounds as coupling partners is reported. Employing a cationic cyclopentadienyliron dicarbonyl complex, this redox neutral process converts simple alkenes into allylic anion equivalents for 1,4-addition into maleimides, acyclic α,β-unsaturated imides, and vinylogous amides. The judicious pairing of pyridine and alkylamine bases with Lewis acid additives allowed each of these classes of substrates to be successfully employed, allowing for the formation of a diverse collection of cyclic and acyclic nitrogen-containing compounds featuring C–C unsaturation. The resulting Michael adducts can be further transformed into a variety of useful scaffolds such as allylated pyrroles, pyrrolidines, and carbocyclic acids.
中文翻译:

通过共轭加成催化烯丙铁亲核试剂实现多种含氮支架的 AC-H 功能化方法
环戊二烯基铁 ( II ) 二羰基络合物能够协调和增强一系列不饱和底物的酸性,已成为一类新型的贱金属衍生催化剂,用于 C-H 官能化。在这篇手稿中,报道了使用含氮 α,β-不饱和羰基化合物作为偶联伙伴对烯丙基 C(sp 3 )-H键进行铁催化的 C-H 官能化。该氧化还原中性过程采用阳离子环戊二烯基铁二羰基络合物,将简单的烯烃转化为烯丙基阴离子等价物,以 1,4-加成反应生成马来酰亚胺、无环 α,β-不饱和酰亚胺和插烯酰胺。吡啶和烷基胺碱与路易斯酸添加剂的明智配对使得每一类底物都能够成功使用,从而形成多种具有 C-C 不饱和特征的环状和无环含氮化合物。所得的迈克尔加合物可以进一步转化为各种有用的支架,例如烯丙基化的吡咯、吡咯烷和碳环酸。
更新日期:2024-05-01
中文翻译:

通过共轭加成催化烯丙铁亲核试剂实现多种含氮支架的 AC-H 功能化方法
环戊二烯基铁 ( II ) 二羰基络合物能够协调和增强一系列不饱和底物的酸性,已成为一类新型的贱金属衍生催化剂,用于 C-H 官能化。在这篇手稿中,报道了使用含氮 α,β-不饱和羰基化合物作为偶联伙伴对烯丙基 C(sp 3 )-H键进行铁催化的 C-H 官能化。该氧化还原中性过程采用阳离子环戊二烯基铁二羰基络合物,将简单的烯烃转化为烯丙基阴离子等价物,以 1,4-加成反应生成马来酰亚胺、无环 α,β-不饱和酰亚胺和插烯酰胺。吡啶和烷基胺碱与路易斯酸添加剂的明智配对使得每一类底物都能够成功使用,从而形成多种具有 C-C 不饱和特征的环状和无环含氮化合物。所得的迈克尔加合物可以进一步转化为各种有用的支架,例如烯丙基化的吡咯、吡咯烷和碳环酸。






























 京公网安备 11010802027423号
京公网安备 11010802027423号