当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Amyloid-like Aggregation of Wheat Gluten and Its Components during Cooking: Mechanisms and Structural Characterization
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2024-05-01 , DOI: 10.1021/acs.jafc.3c09451 Ying Liang 1 , Hao Liu 1 , Yangyi Jie 1 , Mei Liu 2 , Baoshan He 2 , Jinshui Wang 1
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2024-05-01 , DOI: 10.1021/acs.jafc.3c09451 Ying Liang 1 , Hao Liu 1 , Yangyi Jie 1 , Mei Liu 2 , Baoshan He 2 , Jinshui Wang 1
Affiliation
|
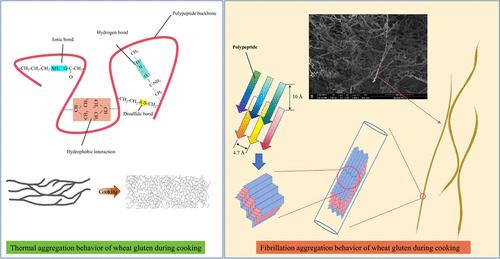
Amyloid-like aggregation widely occurs during the processing and production of natural proteins, with evidence indicating its presence following the thermal processing of wheat gluten. However, significant gaps remain in understanding the underlying fibrillation mechanisms and structural polymorphisms. In this study, the amyloid-like aggregation behavior of wheat gluten and its components (glutenin and gliadin) during cooking was systematically analyzed through physicochemical assessment and structural characterization. The presence of amyloid-like fibrils (AFs) was confirmed using X-ray diffraction and Congo red staining, while Thioflavin T fluorescence revealed different patterns and rates of AFs growth among wheat gluten, glutenin, and gliadin. AFs in gliadin exhibited linear growth curves, while those in gluten and glutenin showed S-shaped curves, with the shortest lag phase and fastest growth rate (t1/2 = 2.11 min) observed in glutenin. Molecular weight analyses revealed AFs primarily in the 10–15 kDa range, shifting to higher weights over time. Glutenin-derived AFs had the smallest ζ-potential value (−19.5 mV) and the most significant size increase post cooking (approximately 400 nm). AFs in gluten involve interchain reorganization, hydrophobic interactions, and conformational transitions, leading to additional cross β-sheets. Atomic force microscopy depicted varying fibril structures during cooking, notably longer, taller, and stiffer AFs from glutenin.
中文翻译:

小麦面筋及其成分在烹饪过程中的类淀粉样聚集:机制和结构表征
淀粉样蛋白样聚集广泛发生在天然蛋白质的加工和生产过程中,有证据表明其在小麦面筋热处理后存在。然而,在理解潜在的颤动机制和结构多态性方面仍然存在重大差距。在这项研究中,通过理化评估和结构表征,系统地分析了小麦面筋及其成分(麦谷蛋白和麦醇溶蛋白)在烹饪过程中的类淀粉样蛋白聚集行为。使用 X 射线衍射和刚果红染色证实了类淀粉样原纤维 (AF) 的存在,而硫磺素 T 荧光揭示了小麦面筋、麦谷蛋白和麦醇溶蛋白中 AF 生长的不同模式和速率。麦醇溶蛋白中的AF呈线性生长曲线,而面筋和麦谷蛋白中的AF呈S形曲线,其中麦谷蛋白的滞后期最短,生长速率最快(t 1/2 = 2.11 min)。分子量分析显示 AF 主要在 10-15 kDa 范围内,随着时间的推移会转向更高的分子量。谷蛋白衍生的 AF 具有最小的 ζ 电位值(−19.5 mV)和烹饪后最显着的尺寸增加(约 400 nm)。面筋中的 AF 涉及链间重组、疏水相互作用和构象转变,从而产生额外的交叉 β 折叠。原子力显微镜描绘了烹饪过程中不同的原纤维结构,特别是来自麦谷蛋白的更长、更高、更硬的纤维结构。
更新日期:2024-05-01
中文翻译:

小麦面筋及其成分在烹饪过程中的类淀粉样聚集:机制和结构表征
淀粉样蛋白样聚集广泛发生在天然蛋白质的加工和生产过程中,有证据表明其在小麦面筋热处理后存在。然而,在理解潜在的颤动机制和结构多态性方面仍然存在重大差距。在这项研究中,通过理化评估和结构表征,系统地分析了小麦面筋及其成分(麦谷蛋白和麦醇溶蛋白)在烹饪过程中的类淀粉样蛋白聚集行为。使用 X 射线衍射和刚果红染色证实了类淀粉样原纤维 (AF) 的存在,而硫磺素 T 荧光揭示了小麦面筋、麦谷蛋白和麦醇溶蛋白中 AF 生长的不同模式和速率。麦醇溶蛋白中的AF呈线性生长曲线,而面筋和麦谷蛋白中的AF呈S形曲线,其中麦谷蛋白的滞后期最短,生长速率最快(t 1/2 = 2.11 min)。分子量分析显示 AF 主要在 10-15 kDa 范围内,随着时间的推移会转向更高的分子量。谷蛋白衍生的 AF 具有最小的 ζ 电位值(−19.5 mV)和烹饪后最显着的尺寸增加(约 400 nm)。面筋中的 AF 涉及链间重组、疏水相互作用和构象转变,从而产生额外的交叉 β 折叠。原子力显微镜描绘了烹饪过程中不同的原纤维结构,特别是来自麦谷蛋白的更长、更高、更硬的纤维结构。






























 京公网安备 11010802027423号
京公网安备 11010802027423号