当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Simultaneously Monitoring Multiple Autophagic Processes and Assessing Autophagic Flux in Single Cells by In Situ Fluorescence Cross-Correlation Spectroscopy
Analytical Chemistry ( IF 7.4 ) Pub Date : 2024-04-22 , DOI: 10.1021/acs.analchem.4c00725 Haohan Song 1 , Chaoqing Dong 1 , Jicun Ren 1
Analytical Chemistry ( IF 7.4 ) Pub Date : 2024-04-22 , DOI: 10.1021/acs.analchem.4c00725 Haohan Song 1 , Chaoqing Dong 1 , Jicun Ren 1
Affiliation
|
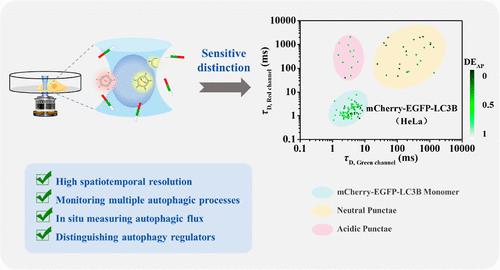
Autophagy is a widely conserved and multistep cellular catabolic process and maintains cellular homeostasis and normal cellular functions via the degradation of some harmful intracellular components. It was reported that high basal autophagic activity may be closely related to tumorigenesis. So far, the fluorescence imaging technique has been widely used to study autophagic processes, but this method is only suitable for distinguishing autophagosomes and autolysosomes. Simultaneously monitoring multiple autophagic processes remains a significant challenge due to the lack of an efficient detection method. Here, we demonstrated a new method for simultaneously monitoring multiple autophagic processes and assessing autophagic flux in single cells based on in situ fluorescence cross-correlation spectroscopy (FCCS). In this study, microtubule-associated protein 1A/1B-light chain 3B (LC3B) was fused with two tandem fluorescent proteins [mCherry red fluorescent protein (mCherry) and enhanced green fluorescent protein (EGFP)] to achieve the simultaneous labeling and distinguishing of multiple autophagic structures based on the differences in characteristic diffusion time (τD). Furthermore, we proposed a new parameter “delivery efficiency of autophagosome (DEAP)” to assess autophagic flux based on the cross correlation (CC) value. Our results demonstrate that FCCS can efficiently distinguish three autophagic structures, assess the induced autophagic flux, and discriminate different autophagy regulators. Compared with the commonly used fluorescence imaging technique, the resolution of FCCS remains unaffected by Brownian motion and fluorescent monomers in the cytoplasm and is well suitable to distinguishing differently colored autophagic structures and monitoring autophagy.
中文翻译:

通过原位荧光互相关光谱同时监测多个自噬过程并评估单细胞中的自噬通量
自噬是一种广泛保守的多步骤细胞分解代谢过程,通过降解一些有害的细胞内成分来维持细胞稳态和正常的细胞功能。据报道,高基础自噬活性可能与肿瘤发生密切相关。迄今为止,荧光成像技术已广泛用于研究自噬过程,但该方法仅适用于区分自噬体和自溶酶体。由于缺乏有效的检测方法,同时监测多个自噬过程仍然是一个重大挑战。在这里,我们展示了一种基于原位荧光互相关光谱(FCCS)同时监测多个自噬过程并评估单细胞自噬通量的新方法。本研究将微管相关蛋白1A/1B-轻链3B(LC3B)与两种串联荧光蛋白[mCherry红色荧光蛋白(mCherry)和增强型绿色荧光蛋白(EGFP)]融合,实现了微管相关蛋白1A/1B-轻链3B(LC3B)的同时标记和区分。基于特征扩散时间(τ D)差异的多种自噬结构。此外,我们提出了一个新参数“自噬体的传递效率(DE AP)”来基于互相关(CC)值评估自噬通量。我们的结果表明,FCCS 可以有效地区分三种自噬结构,评估诱导的自噬通量,并区分不同的自噬调节因子。与常用的荧光成像技术相比,FCCS的分辨率不受布朗运动和细胞质中荧光单体的影响,非常适合区分不同颜色的自噬结构和监测自噬。
更新日期:2024-04-22
中文翻译:

通过原位荧光互相关光谱同时监测多个自噬过程并评估单细胞中的自噬通量
自噬是一种广泛保守的多步骤细胞分解代谢过程,通过降解一些有害的细胞内成分来维持细胞稳态和正常的细胞功能。据报道,高基础自噬活性可能与肿瘤发生密切相关。迄今为止,荧光成像技术已广泛用于研究自噬过程,但该方法仅适用于区分自噬体和自溶酶体。由于缺乏有效的检测方法,同时监测多个自噬过程仍然是一个重大挑战。在这里,我们展示了一种基于原位荧光互相关光谱(FCCS)同时监测多个自噬过程并评估单细胞自噬通量的新方法。本研究将微管相关蛋白1A/1B-轻链3B(LC3B)与两种串联荧光蛋白[mCherry红色荧光蛋白(mCherry)和增强型绿色荧光蛋白(EGFP)]融合,实现了微管相关蛋白1A/1B-轻链3B(LC3B)的同时标记和区分。基于特征扩散时间(τ D)差异的多种自噬结构。此外,我们提出了一个新参数“自噬体的传递效率(DE AP)”来基于互相关(CC)值评估自噬通量。我们的结果表明,FCCS 可以有效地区分三种自噬结构,评估诱导的自噬通量,并区分不同的自噬调节因子。与常用的荧光成像技术相比,FCCS的分辨率不受布朗运动和细胞质中荧光单体的影响,非常适合区分不同颜色的自噬结构和监测自噬。



























 京公网安备 11010802027423号
京公网安备 11010802027423号