Cell ( IF 64.5 ) Pub Date : 2024-04-25 , DOI: 10.1016/j.cell.2024.03.022 Benjamin L. Lampson , Ana S. Ramίrez , Marta Baro , Lixia He , Mudra Hegde , Vidyasagar Koduri , Jamie L. Pfaff , Ruth E. Hanna , Julia Kowal , Nitin H. Shirole , Yanfeng He , John G. Doench , Joseph N. Contessa , Kaspar P. Locher , William G. Kaelin
|
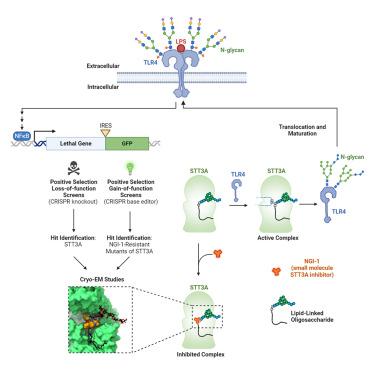
Nuclear factor κB (NF-κB) plays roles in various diseases. Many inflammatory signals, such as circulating lipopolysaccharides (LPSs), activate NF-κB via specific receptors. Using whole-genome CRISPR-Cas9 screens of LPS-treated cells that express an NF-κB-driven suicide gene, we discovered that the LPS receptor Toll-like receptor 4 (TLR4) is specifically dependent on the oligosaccharyltransferase complex OST-A for N-glycosylation and cell-surface localization. The tool compound NGI-1 inhibits OST complexes in vivo, but the underlying molecular mechanism remained unknown. We did a CRISPR base-editor screen for NGI-1-resistant variants of STT3A, the catalytic subunit of OST-A. These variants, in conjunction with cryoelectron microscopy studies, revealed that NGI-1 binds the catalytic site of STT3A, where it traps a molecule of the donor substrate dolichyl-PP-GlcNAc2-Man9-Glc3, suggesting an uncompetitive inhibition mechanism. Our results provide a rationale for and an initial step toward the development of STT3A-specific inhibitors and illustrate the power of contemporaneous base-editor and structural studies to define drug mechanism of action.
中文翻译:

正选 CRISPR 筛选揭示了向 NF-κB 发出炎症信号所需的寡糖转移酶中的可药物口袋
核因子κB (NF-κB) 在多种疾病中发挥作用。许多炎症信号,例如循环脂多糖 (LPS),通过特定受体激活 NF-κB。通过对表达 NF-κB 驱动自杀基因的 LPS 处理细胞进行全基因组 CRISPR-Cas9 筛选,我们发现 LPS 受体 Toll 样受体 4 (TLR4) 特异性依赖于寡糖转移酶复合物 OST- A -糖基化和细胞表面定位。工具化合物NGI-1在体内抑制OST复合物,但潜在的分子机制仍不清楚。我们对 STT3A(OST-A 的催化亚基)的 NGI-1 抗性变体进行了 CRISPR 碱基编辑器筛选。这些变体与冷冻电子显微镜研究相结合,揭示 NGI-1 结合 STT3A 的催化位点,在该位点捕获供体底物 dolichyl-PP-GlcNAc 2 -Man 9 -Glc 3的分子,表明非竞争性抑制机制。我们的结果为开发 STT3A 特异性抑制剂提供了基本原理和第一步,并说明了同期碱基编辑和结构研究在定义药物作用机制方面的力量。



























 京公网安备 11010802027423号
京公网安备 11010802027423号