当前位置:
X-MOL 学术
›
Catal. Today
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tailoring the hydrophobicity of nickel hydroxide for selective electrooxidation of methane to methanol and ethanol
Catalysis Today ( IF 5.3 ) Pub Date : 2024-04-17 , DOI: 10.1016/j.cattod.2024.114734 Ru-Meng Wang , Hao Tian , Lei Bian , Yu Bai , Shi-Bing Liu , Zhi Ma , Zhong-Li Wang
Catalysis Today ( IF 5.3 ) Pub Date : 2024-04-17 , DOI: 10.1016/j.cattod.2024.114734 Ru-Meng Wang , Hao Tian , Lei Bian , Yu Bai , Shi-Bing Liu , Zhi Ma , Zhong-Li Wang
|
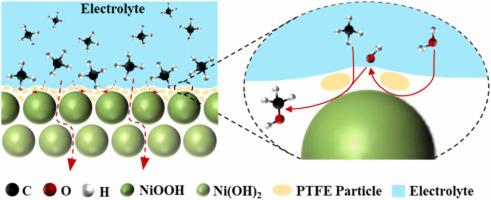
Electrocatalytic oxidation of methane (CH) to value-added chemicals under mild conditions derived by renewable electricity is an attractive approach to directly use natural gas as a hydrocarbon feedstock. Herein, we report a novel composite electrode constructed by uniformly dispersing hydrophobic polytetrafluoroethylene (PTFE) polymer on the surface of Ni(OH) nanosheets catalyst for selective electrooxidation of CH to methanol (CHOH) and ethanol (CHCHOH). This hydrophobic composite electrode can establish a directional transport channel for CH and effectively enhance the enrichment of CH on the electrode surface, forming a rich solid-liquid-gas three-phase reaction interface. The optimized 6 %PTFE-Ni(OH) electrode can achieve the highest alcohols’ Faradaic efficiency (FE) of 75.8 %, which is 21 times higher than that of the hydrophilic Ni(OH)/NF electrode, and the highest concentration of alcohols can reach 9.00 mM. In addition, mechanistic analysis reveals that Ni(OH) is oxidized to NiOOH, which can oxidizes water to *OH, and then *OH activates and oxidizes CH to *CH and CHOH. In addition, the formation mechanism of CHCHOH is only formed by coupling *OCH with CH activated on the catalyst surface. This work provides a feasible approach to tailor the hydrophilicity of catalyst to improve the efficiency of CH oxidation.
中文翻译:

定制氢氧化镍的疏水性用于甲烷选择性电氧化为甲醇和乙醇
在可再生电力产生的温和条件下将甲烷(CH)电催化氧化为增值化学品是直接使用天然气作为碳氢化合物原料的一种有吸引力的方法。在此,我们报道了一种新型复合电极,该电极由均匀分散在 Ni(OH) 纳米片催化剂表面的疏水性聚四氟乙烯 (PTFE) 聚合物构成,用于 CH 选择性电氧化为甲醇 (CHOH) 和乙醇 (CHCHOH)。这种疏水性复合电极可以建立CH的定向传输通道,有效增强CH在电极表面的富集,形成丰富的固-液-气三相反应界面。优化后的6%PTFE-Ni(OH)电极可以实现最高的醇类法拉第效率(FE)75.8%,比亲水性Ni(OH)/NF电极高21倍,并且醇类浓度最高可以达到9.00 mM。此外,机理分析表明Ni(OH)被氧化为NiOOH,NiOOH可将水氧化为*OH,然后*OH活化并氧化CH为*CH和CHOH。此外,CHCHOH的形成机理仅是通过*OCH与催化剂表面活化的CH偶联而形成。这项工作提供了一种调整催化剂亲水性以提高 CH 氧化效率的可行方法。
更新日期:2024-04-17
中文翻译:

定制氢氧化镍的疏水性用于甲烷选择性电氧化为甲醇和乙醇
在可再生电力产生的温和条件下将甲烷(CH)电催化氧化为增值化学品是直接使用天然气作为碳氢化合物原料的一种有吸引力的方法。在此,我们报道了一种新型复合电极,该电极由均匀分散在 Ni(OH) 纳米片催化剂表面的疏水性聚四氟乙烯 (PTFE) 聚合物构成,用于 CH 选择性电氧化为甲醇 (CHOH) 和乙醇 (CHCHOH)。这种疏水性复合电极可以建立CH的定向传输通道,有效增强CH在电极表面的富集,形成丰富的固-液-气三相反应界面。优化后的6%PTFE-Ni(OH)电极可以实现最高的醇类法拉第效率(FE)75.8%,比亲水性Ni(OH)/NF电极高21倍,并且醇类浓度最高可以达到9.00 mM。此外,机理分析表明Ni(OH)被氧化为NiOOH,NiOOH可将水氧化为*OH,然后*OH活化并氧化CH为*CH和CHOH。此外,CHCHOH的形成机理仅是通过*OCH与催化剂表面活化的CH偶联而形成。这项工作提供了一种调整催化剂亲水性以提高 CH 氧化效率的可行方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号