当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Machine Learning for Experimental Reactivity of a Set of Metal Clusters toward C–H Activation
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-23 , DOI: 10.1021/jacs.4c00501 Xi-Guan Zhao 1, 2, 3 , Qi Yang 1, 2, 3 , Ying Xu 1, 2, 3 , Qing-Yu Liu 1, 3 , Zi-Yu Li 1, 3 , Xiao-Xiao Liu 1, 2, 3 , Yan-Xia Zhao 1, 3 , Sheng-Gui He 1, 2, 3
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-23 , DOI: 10.1021/jacs.4c00501 Xi-Guan Zhao 1, 2, 3 , Qi Yang 1, 2, 3 , Ying Xu 1, 2, 3 , Qing-Yu Liu 1, 3 , Zi-Yu Li 1, 3 , Xiao-Xiao Liu 1, 2, 3 , Yan-Xia Zhao 1, 3 , Sheng-Gui He 1, 2, 3
Affiliation
|
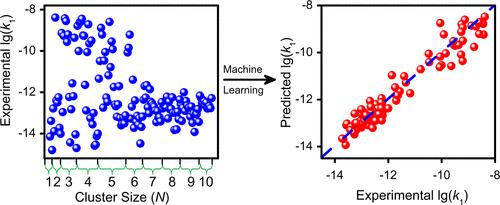
Understanding the mechanisms of C–H activation of alkanes is a very important research topic. The reactions of metal clusters with alkanes have been extensively studied to reveal the electronic features governing C–H activation, while the experimental cluster reactivity was qualitatively interpreted case by case in the literature. Herein, we prepared and mass-selected over 100 rhodium-based clusters (RhxVyOz– and RhxCoyOz–) to react with light alkanes, enabling the determination of reaction rate constants spanning six orders of magnitude. A satisfactory model being able to quantitatively describe the rate data in terms of multiple cluster electronic features (average electron occupancy of valence s orbitals, the minimum natural charge on the metal atom, cluster polarizability, and energy gap involved in the agostic interaction) has been constructed through a machine learning approach. This study demonstrates that the general mechanisms governing the very important process of C–H activation by diverse metal centers can be discovered by interpreting experimental data with artificial intelligence.
中文翻译:

一组金属簇对 C-H 激活的实验反应性的机器学习
了解烷烃的C-H活化机制是一个非常重要的研究课题。金属簇与烷烃的反应已被广泛研究,以揭示控制 C-H 活化的电子特征,而实验簇反应性在文献中逐个案例进行了定性解释。在此,我们制备并大规模选择了 100 多个铑基团簇(Rh x V y O z –和 Rh x Co y O z –)来与轻烷烃发生反应,从而能够确定跨越六个数量级的反应速率常数。一个令人满意的模型能够定量地描述多团簇电子特征(价轨道的平均电子占据率、金属原子上的最小自然电荷、团簇极化率和参与失能相互作用的能隙)的速率数据。通过机器学习方法构建。这项研究表明,通过人工智能解释实验数据,可以发现控制不同金属中心 C-H 激活这一非常重要过程的一般机制。
更新日期:2024-04-24
中文翻译:

一组金属簇对 C-H 激活的实验反应性的机器学习
了解烷烃的C-H活化机制是一个非常重要的研究课题。金属簇与烷烃的反应已被广泛研究,以揭示控制 C-H 活化的电子特征,而实验簇反应性在文献中逐个案例进行了定性解释。在此,我们制备并大规模选择了 100 多个铑基团簇(Rh x V y O z –和 Rh x Co y O z –)来与轻烷烃发生反应,从而能够确定跨越六个数量级的反应速率常数。一个令人满意的模型能够定量地描述多团簇电子特征(价轨道的平均电子占据率、金属原子上的最小自然电荷、团簇极化率和参与失能相互作用的能隙)的速率数据。通过机器学习方法构建。这项研究表明,通过人工智能解释实验数据,可以发现控制不同金属中心 C-H 激活这一非常重要过程的一般机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号