当前位置:
X-MOL 学术
›
Electrochim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Study of the performance of glucose anomers in a hydroxide anion exchange membrane electrolyzer operating with pulse electrodeposited gold for paired electrosynthesis
Electrochimica Acta ( IF 6.6 ) Pub Date : 2024-04-15 , DOI: 10.1016/j.electacta.2024.144275 Zahra Hagheh Kavousi , Amira Ben Abderrahmane , Massomeh Ghorbanloo , Sophie Tingry , David Cornu , Mikhael Bechelany , Yaovi Holade
Electrochimica Acta ( IF 6.6 ) Pub Date : 2024-04-15 , DOI: 10.1016/j.electacta.2024.144275 Zahra Hagheh Kavousi , Amira Ben Abderrahmane , Massomeh Ghorbanloo , Sophie Tingry , David Cornu , Mikhael Bechelany , Yaovi Holade
|
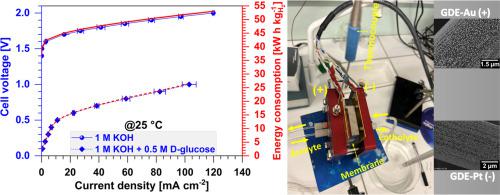
Electroconversion (also known as electroreforming) of biomass-derived compounds is currently attracting considerable interest, with the aim of lowering cell voltage during electrolysis to co-produce a number of decarbonized energy carriers or chemical intermediates (hydrogen, ammonia, gluconate, etc.). Naturally, -glucose, which represents a study model for the electro-valorization of cellulosic biomass into value-added chemicals such as gluconate, is in fact a mixture of two anomers called α-- and β--glucose. The β--glucose anomer is the monomer unit of cellulose, while the α--glucose anomer is the monomer unit of starch. In this contribution, we therefore investigate whether or not the nature of the glucose substrate can influence electrolysis cell performance, or whether the use of -glucose alone can represent the true picture of biomass feeding an electrolysis cell. Free-standing electrocatalysts ready for use in a membrane-electrode assembly were synthesized using a pulsed electrodeposition methodology to control the deposition of gold and platinum particles on the microfibers of a low-metal gas diffusion electrode (GDE). We developed GDE-Au (82 μg cm, 0.88 wt%) to catalyze the selective electrooxidation of glucose into gluconate, and GDE-Pt (33 μg cm, 0.36 wt%) to catalyze the hydrogen evolution reaction (HER). While half-cell studies showed no significant difference, for the glucose-fed electrolysis cell, α--glucose leads to a much higher current density compared to -glucose and β--glucose for cell voltages above 0.5 V, leading to 85.6 ± 17.1, 44.3 ± 8.8 and 30.5 ± 0.8 mA cm for α--glucose, β--glucose, and -glucose, respectively, at 1 V.
中文翻译:

脉冲电沉积金配对电合成氢氧阴离子交换膜电解槽中葡萄糖端基异构体的性能研究
生物质衍生化合物的电转化(也称为电重整)目前引起了相当大的兴趣,其目的是在电解过程中降低电池电压,以共同生产许多脱碳能量载体或化学中间体(氢、氨、葡萄糖酸盐等)。 。当然,β-葡萄糖代表了将纤维素生物质电增值为葡萄糖酸等增值化学品的研究模型,它实际上是两种称为 α- 和 β- 葡萄糖的异头物的混合物。 β-葡萄糖端基异构体是纤维素的单体单元,而α-葡萄糖端基异构体是淀粉的单体单元。因此,在本贡献中,我们研究了葡萄糖底物的性质是否会影响电解池的性能,或者单独使用β-葡萄糖是否可以代表电解池供给生物质的真实情况。使用脉冲电沉积方法合成了可用于膜电极组件的独立式电催化剂,以控制金和铂颗粒在低金属气体扩散电极(GDE)微纤维上的沉积。我们开发了GDE-Au(82 μg cm,0.88 wt%)来催化葡萄糖选择性电氧化成葡萄糖酸,以及GDE-Pt(33 μg cm,0.36 wt%)来催化析氢反应(HER)。虽然半电池研究显示没有显着差异,但对于葡萄糖供给的电解池,当电池电压高于 0.5 V 时,α-葡萄糖比 β-葡萄糖和 β-葡萄糖产生更高的电流密度,导致 85.6 ± 17.1 1 V 时,α-葡萄糖、β-葡萄糖和 β-葡萄糖分别为 44.3 ± 8.8 和 30.5 ± 0.8 mA cm。
更新日期:2024-04-15
中文翻译:

脉冲电沉积金配对电合成氢氧阴离子交换膜电解槽中葡萄糖端基异构体的性能研究
生物质衍生化合物的电转化(也称为电重整)目前引起了相当大的兴趣,其目的是在电解过程中降低电池电压,以共同生产许多脱碳能量载体或化学中间体(氢、氨、葡萄糖酸盐等)。 。当然,β-葡萄糖代表了将纤维素生物质电增值为葡萄糖酸等增值化学品的研究模型,它实际上是两种称为 α- 和 β- 葡萄糖的异头物的混合物。 β-葡萄糖端基异构体是纤维素的单体单元,而α-葡萄糖端基异构体是淀粉的单体单元。因此,在本贡献中,我们研究了葡萄糖底物的性质是否会影响电解池的性能,或者单独使用β-葡萄糖是否可以代表电解池供给生物质的真实情况。使用脉冲电沉积方法合成了可用于膜电极组件的独立式电催化剂,以控制金和铂颗粒在低金属气体扩散电极(GDE)微纤维上的沉积。我们开发了GDE-Au(82 μg cm,0.88 wt%)来催化葡萄糖选择性电氧化成葡萄糖酸,以及GDE-Pt(33 μg cm,0.36 wt%)来催化析氢反应(HER)。虽然半电池研究显示没有显着差异,但对于葡萄糖供给的电解池,当电池电压高于 0.5 V 时,α-葡萄糖比 β-葡萄糖和 β-葡萄糖产生更高的电流密度,导致 85.6 ± 17.1 1 V 时,α-葡萄糖、β-葡萄糖和 β-葡萄糖分别为 44.3 ± 8.8 和 30.5 ± 0.8 mA cm。



























 京公网安备 11010802027423号
京公网安备 11010802027423号