当前位置:
X-MOL 学术
›
Cell Calcium
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Simultaneous TIRF imaging of subplasmalemmal Ca2+ dynamics and granule fusions in insulin-secreting INS-1 cells reveals coexistent synchronized and asynchronous release
Cell Calcium ( IF 4 ) Pub Date : 2024-04-08 , DOI: 10.1016/j.ceca.2024.102883 Charlotte Suckert , Carolin Zosel , Michael Schaefer
Cell Calcium ( IF 4 ) Pub Date : 2024-04-08 , DOI: 10.1016/j.ceca.2024.102883 Charlotte Suckert , Carolin Zosel , Michael Schaefer
|
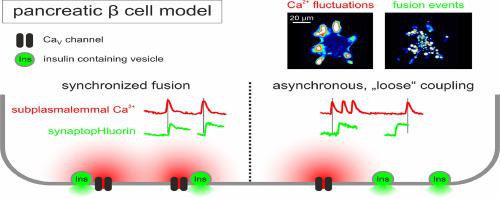
The basal and glucose-induced insulin secretion from pancreatic beta cells is a tightly regulated process that is triggered in a Ca-dependent fashion and further positively modulated by substances that raise intracellular levels of adenosine 3′,5′-cyclic monophosphate (cAMP) or by certain antidiabetic drugs. In a previous study, we have temporally resolved the subplasmalemmal [Ca] dynamics in beta cells that are characterized by trains of sharply delimited spikes, reaching peak values up to 5 µM. Applying total internal reflection fluorescence (TIRF) microscopy and synaptopHluorin to visualize fusion events of individual granules, we found that several fusion events can coincide within 50 to 150 ms. To test whether subplasmalemmal [Ca] microdomains around single or clustered Ca channels may cause a synchronized release of insulin-containing vesicles, we applied simultaneous dual-color TIRF microscopy and monitored Ca fluctuations and exocytotic events in INS-1 cells at high frame rates. The results indicate that fusions can be triggered by subplasmalemmal Ca spiking. This, however, does account for a minority of fusion events. About 90 %-95 % of fusion events either happen between Ca spikes or incidentally overlap with subplasmalemmal Ca spikes. We conclude that only a fraction of exocytotic events in glucose-induced and tolbutamide- or forskolin-enhanced insulin release from INS-1 cells is tightly coupled to Ca microdomains around voltage-gated Ca channels.
中文翻译:

胰岛素分泌 INS-1 细胞中质膜下 Ca2+ 动力学和颗粒融合的同步 TIRF 成像揭示了同步和异步释放共存
胰腺β细胞的基础和葡萄糖诱导的胰岛素分泌是一个严格调节的过程,该过程以Ca依赖性方式触发,并受到提高细胞内腺苷3',5'-环单磷酸(cAMP)或通过某些抗糖尿病药物。在之前的一项研究中,我们暂时解决了 β 细胞中质膜下 [Ca] 动力学的问题,其特征是一系列尖锐的尖峰,达到峰值高达 5 µM。应用全内反射荧光 (TIRF) 显微镜和 synaptopHluorin 来可视化单个颗粒的融合事件,我们发现多个融合事件可以在 50 至 150 毫秒内同时发生。为了测试单个或簇状 Ca 通道周围的质膜下 [Ca] 微域是否可能导致含胰岛素囊泡的同步释放,我们应用同步双色 TIRF 显微镜并以高帧速率监测 INS-1 细胞中的 Ca 波动和胞吐事件。结果表明融合可以由质膜下 Ca 尖峰触发。然而,这确实解释了少数聚变事件。大约 90%-95% 的融合事件要么发生在 Ca 尖峰之间,要么偶然与质膜下 Ca 尖峰重叠。我们得出的结论是,在葡萄糖诱导和甲苯磺丁脲或毛喉素增强的 INS-1 细胞胰岛素释放中,只有一小部分胞吐事件与电压门控 Ca 通道周围的 Ca 微域紧密耦合。
更新日期:2024-04-08
中文翻译:

胰岛素分泌 INS-1 细胞中质膜下 Ca2+ 动力学和颗粒融合的同步 TIRF 成像揭示了同步和异步释放共存
胰腺β细胞的基础和葡萄糖诱导的胰岛素分泌是一个严格调节的过程,该过程以Ca依赖性方式触发,并受到提高细胞内腺苷3',5'-环单磷酸(cAMP)或通过某些抗糖尿病药物。在之前的一项研究中,我们暂时解决了 β 细胞中质膜下 [Ca] 动力学的问题,其特征是一系列尖锐的尖峰,达到峰值高达 5 µM。应用全内反射荧光 (TIRF) 显微镜和 synaptopHluorin 来可视化单个颗粒的融合事件,我们发现多个融合事件可以在 50 至 150 毫秒内同时发生。为了测试单个或簇状 Ca 通道周围的质膜下 [Ca] 微域是否可能导致含胰岛素囊泡的同步释放,我们应用同步双色 TIRF 显微镜并以高帧速率监测 INS-1 细胞中的 Ca 波动和胞吐事件。结果表明融合可以由质膜下 Ca 尖峰触发。然而,这确实解释了少数聚变事件。大约 90%-95% 的融合事件要么发生在 Ca 尖峰之间,要么偶然与质膜下 Ca 尖峰重叠。我们得出的结论是,在葡萄糖诱导和甲苯磺丁脲或毛喉素增强的 INS-1 细胞胰岛素释放中,只有一小部分胞吐事件与电压门控 Ca 通道周围的 Ca 微域紧密耦合。



























 京公网安备 11010802027423号
京公网安备 11010802027423号