Chemical Engineering Journal ( IF 15.1 ) Pub Date : 2024-04-19 , DOI: 10.1016/j.cej.2024.151464 Xingyuan Feng , Hao Chen , Qi Xue , Changluo Su , Yuanzhen Zhou
|
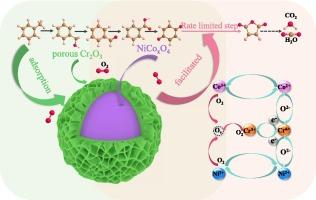
Bimetallic-based spinels are potential materials for toluene oxidation ascribing to their remarkable stability and oxidation activity. However, the insufficient toluene adsorption capacity and limited reactive oxygen species severely restrict their low-temperature catalytic reactivity. This study reports a facile two-step solvothermal strategy for the synthesis of core–shell structured CrOx/NiCoxO4 catalyst, which exhibits excellent toluene oxidation performance with T50 and T90 values of 187 and 218 °C, respectively, and the toluene conversion remains above 90 % at 218 °C for 20 h. The in-situ DRIFTs indicate that the oxidation of toluene to CO2 and H2O follows Mars-van Krevelen (MvK) mechanism, and the rate limiting step is the oxidation of quinone to maleic anhydride. The strong interaction between core NiCoxO4 and shell CrOx regulates the morphology of CrOx from particles to porous flocculent structure, which increases the porosity of catalyst and promotes the adsorption of toluene for further oxidation. Moreover, the interaction promotes the charge transfer from NiCoxO4 to CrOx, which increases the adsorbed oxygen species contents and the redox capability of the catalyst. A further density functional theory (DFT) calculation confirms that the core NiCoxO4 is the main active component, where the oxygen vacancy formation energy of NiCoxO4 and CrOx decrease from 2.43 and 3.85 eV to 0.31 and 1.52 eV, while the toluene adsorption energy decrease from −0.04 and −0.94 eV to −5.29, and −4.48 eV, respectively, which evidently demonstrates that the formation of core–shell structured CrOx/NiCoxO4 would significantly promote the adsorption and oxidation of toluene, thereby exhibiting excellent low temperature toluene oxidation reactivity.
中文翻译:

构建用于甲苯低温催化氧化的核壳结构 CrOx/NiCoxO4 催化剂:了解核壳相互作用的反应促进机制
双金属尖晶石因其卓越的稳定性和氧化活性而成为甲苯氧化的潜在材料。然而,甲苯吸附能力不足和活性氧物种有限严重限制了其低温催化反应活性。本研究报告了一种简便的两步溶剂热策略,用于合成核壳结构的 CrO x /NiCo x O 4催化剂,该催化剂表现出优异的甲苯氧化性能,T 50和 T 90值分别为 187 和 218 °C,并且在 218 °C 下 20 小时,甲苯转化率保持在 90% 以上。原位DRIFT表明,甲苯氧化成CO 2和H 2 O遵循Mars-van Krevelen (MvK)机理,限速步骤是醌氧化成马来酸酐。核NiCo x O 4与壳CrO x之间的强相互作用调节CrO x的形貌从颗粒到多孔絮状结构,增加了催化剂的孔隙率,促进甲苯的吸附进一步氧化。此外,这种相互作用促进了电荷从NiCo x O 4到CrO x的转移,从而增加了吸附氧含量和催化剂的氧化还原能力。进一步的密度泛函理论(DFT)计算证实核心NiCo x O 4是主要活性成分,其中NiCo x O 4和CrO x的氧空位形成能从2.43和3.85 eV下降到0.31和1.52 eV,而甲苯吸附能分别从-0.04和-0.94 eV下降到-5.29和-4.48 eV,这明显表明核壳结构CrO x /NiCo x O 4的形成会显着促进甲苯的吸附和氧化,从而表现出优异的低温甲苯氧化反应活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号