当前位置:
X-MOL 学术
›
Food Hydrocoll.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Inhibition of the interactions of myofibrillar proteins with gallic acid by β-cyclodextrin-metal-organic frameworks improves gel quality under oxidative stress
Food Hydrocolloids ( IF 10.7 ) Pub Date : 2024-04-07 , DOI: 10.1016/j.foodhyd.2024.110065 Jinyu Chen , Beibei Jia , Siyang Wang , Zhuoling Li , Zhirui Ji , Ximing Li , Zijian Wu
Food Hydrocolloids ( IF 10.7 ) Pub Date : 2024-04-07 , DOI: 10.1016/j.foodhyd.2024.110065 Jinyu Chen , Beibei Jia , Siyang Wang , Zhuoling Li , Zhirui Ji , Ximing Li , Zijian Wu
|
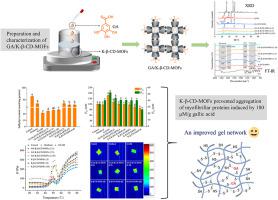
Generally, high levels of natural phenolic antioxidants, which are needed in meat products to exert equivalent antioxidant effects as synthetic antioxidants, can interact with myofibrillar proteins and cause aggregation of the proteins, giving rise to undesirable deterioration of gel quality. In this study, β-cyclodextrin-metal-organic frameworks (K-β-CD-MOFs) were synthesized based on β-CD and K ions using methanol vapor diffusion method, and used to inhibit excessive covalent and non-covalent interactions between gallic acid (GA) and myofibrillar proteins under oxidative stress without degrading the phenolic antioxidant activity. Results of zeta potential, scanning electron microscope (SEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), and differential scanning calorimetry (DSC) provided evidence for the formation of GA/K-β-CD-MOFs inclusion complexes. From sulfydryl content, surface hydrophobicity, tryptophan fluorescence, turbidity and particle size analyses, the presence of K-β-CD-MOFs dose-dependently prevented the sulfydryl loss, unfolding of the proteins and formation of insoluble aggregates caused by GA (180 μM/g protein). Dynamic rheology, cooking loss, gel strength, water distribution, microstructure and Raman spectrum were further tested to evaluate effects of K-β-CD-MOFs on the qualities of GA-treated myofibrillar protein gel. Results indicated that more proteins were involved in the formation of a more ordered and homogeneous gel structure with higher gel elasticity and strength, more immobilized water and decreased cooking loss. The overall gel properties of myofibrillar proteins were improved. K-β-CD-MOFs ameliorated the instability of protein structure, manifested as increase in β-sheet content and inter-chain hydrogen bonding and decrease in hydrophobic forces ( < 0.05). This paper provides a novel method to increase the loading amount of phenolic antioxidants without jeopardizing the gel quality of meat products.
中文翻译:

通过β-环糊精-金属-有机框架抑制肌原纤维蛋白与没食子酸的相互作用,可改善氧化应激下的凝胶质量
一般来说,肉制品中需要高含量的天然酚类抗氧化剂才能发挥与合成抗氧化剂相当的抗氧化作用,但它们会与肌原纤维蛋白相互作用并导致蛋白质聚集,从而导致凝胶质量发生不良恶化。本研究以β-CD和K离子为基础,采用甲醇蒸气扩散法合成了β-环糊精-金属有机框架(K-β-CD-MOFs),并用于抑制没食子之间过度的共价和非共价相互作用。酸(GA)和肌原纤维蛋白在氧化应激下不降低酚类抗氧化活性。 Zeta电位、扫描电子显微镜(SEM)、X射线衍射(XRD)、傅里叶变换红外光谱(FT-IR)和差示扫描量热法(DSC)的结果为GA/K-β-CD的形成提供了证据-MOFs包合物。从巯基含量、表面疏水性、色氨酸荧光、浊度和粒径分析来看,K-β-CD-MOF 的存在剂量依赖性地阻止了 GA(180 μM/克蛋白质)。进一步测试动态流变学、蒸煮损失、凝胶强度、水分分布、微观结构和拉曼光谱,以评估 K-β-CD-MOFs 对 GA 处理的肌原纤维蛋白凝胶质量的影响。结果表明,更多的蛋白质参与形成更有序和均匀的凝胶结构,具有更高的凝胶弹性和强度、更多的固定水和减少的蒸煮损失。肌原纤维蛋白的整体凝胶特性得到改善。 K-β-CD-MOFs改善了蛋白质结构的不稳定性,表现为β-折叠含量和链间氢键增加以及疏水力降低(<0.05)。本文提供了一种在不损害肉制品凝胶质量的情况下增加酚类抗氧化剂负载量的新方法。
更新日期:2024-04-07
中文翻译:

通过β-环糊精-金属-有机框架抑制肌原纤维蛋白与没食子酸的相互作用,可改善氧化应激下的凝胶质量
一般来说,肉制品中需要高含量的天然酚类抗氧化剂才能发挥与合成抗氧化剂相当的抗氧化作用,但它们会与肌原纤维蛋白相互作用并导致蛋白质聚集,从而导致凝胶质量发生不良恶化。本研究以β-CD和K离子为基础,采用甲醇蒸气扩散法合成了β-环糊精-金属有机框架(K-β-CD-MOFs),并用于抑制没食子之间过度的共价和非共价相互作用。酸(GA)和肌原纤维蛋白在氧化应激下不降低酚类抗氧化活性。 Zeta电位、扫描电子显微镜(SEM)、X射线衍射(XRD)、傅里叶变换红外光谱(FT-IR)和差示扫描量热法(DSC)的结果为GA/K-β-CD的形成提供了证据-MOFs包合物。从巯基含量、表面疏水性、色氨酸荧光、浊度和粒径分析来看,K-β-CD-MOF 的存在剂量依赖性地阻止了 GA(180 μM/克蛋白质)。进一步测试动态流变学、蒸煮损失、凝胶强度、水分分布、微观结构和拉曼光谱,以评估 K-β-CD-MOFs 对 GA 处理的肌原纤维蛋白凝胶质量的影响。结果表明,更多的蛋白质参与形成更有序和均匀的凝胶结构,具有更高的凝胶弹性和强度、更多的固定水和减少的蒸煮损失。肌原纤维蛋白的整体凝胶特性得到改善。 K-β-CD-MOFs改善了蛋白质结构的不稳定性,表现为β-折叠含量和链间氢键增加以及疏水力降低(<0.05)。本文提供了一种在不损害肉制品凝胶质量的情况下增加酚类抗氧化剂负载量的新方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号