当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ce4+/Ce3+ Redox-Promoted Electron Transfer for Efficient Neutral H2O2 Electrosynthesis from Two-Electron Oxygen Reduction
ACS Catalysis ( IF 12.9 ) Pub Date : 2024-04-18 , DOI: 10.1021/acscatal.4c00625 Sohee Kim 1 , Young Jin Sa 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2024-04-18 , DOI: 10.1021/acscatal.4c00625 Sohee Kim 1 , Young Jin Sa 1
Affiliation
|
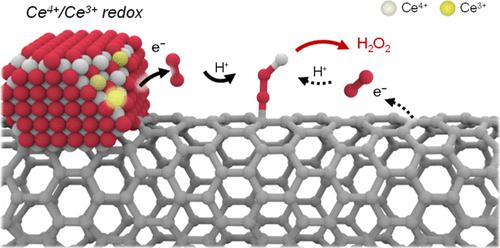
Electrochemical production of H2O2 via a 2-electron oxygen reduction reaction (2e– ORR) provides a clean alternative to the traditional industrial process. H2O2 electrosynthesis in noncaustic neutral electrolytes is desirable for broader applications; however, it requires larger overpotentials compared to those in alkaline electrolytes where a high 2e– ORR activity can be achieved even with metal-free carbon electrocatalysts. Although ceria has been widely adopted as a catalytic promoter in thermo- and electrocatalytic reactions, its roles in enhancing the neutral 2e– ORR have seldom been explored and remain unclear. In this work, we prepared ceria nanoparticles supported on carbon nanotube (CeOx/CNT) composite catalysts and investigated ceria’s promotional effect on the neutral 2e– ORR. The optimal CeOx/CNT catalyst demonstrated a 1.5-fold increase in ORR activity compared to CNT alone, with high H2O2 selectivity over 87%. Electrochemical impedance spectroscopy indicated that the activity improvement correlated with an enhanced electron transfer (ET) rate. In situ X-ray absorption near-edge structure analysis revealed a counterintuitive decrease in the Ce3+/Ce4+ ratio as the applied potential was lowered. This finding suggested ET from Ce3+ to O2, as supported by further electrochemical measurements. In addition, in situ Raman spectroscopy indicated the participation of the CNT in the electrocatalysis. The combination of electrochemical tests and in situ spectroscopies proposes a cascade reaction pathway, where O2 is initially reduced by Ce3+ and subsequently adsorbed onto the active carbon sites.
中文翻译:

Ce4+/Ce3+ 氧化还原促进电子转移,用于双电子氧还原高效中性 H2O2 电合成
通过 2 电子氧还原反应 (2e – ORR)电化学生产 H 2 O 2提供了传统工业过程的清洁替代方案。非苛性中性电解质中的H 2 O 2电合成具有更广泛的应用前景;然而,与碱性电解质相比,它需要更大的过电势,即使使用不含金属的碳电催化剂也可以实现高 2e – ORR 活性。尽管二氧化铈已被广泛用作热催化和电催化反应中的催化促进剂,但其在增强中性 2e – ORR 中的作用很少被探索且仍不清楚。在这项工作中,我们制备了负载在碳纳米管(CeO x /CNT)复合催化剂上的二氧化铈纳米颗粒,并研究了二氧化铈对中性2e - ORR的促进作用。与单独的CNT相比,最佳的CeO x /CNT催化剂的ORR活性提高了1.5倍,并且H 2 O 2选择性超过87%。电化学阻抗谱表明活性的改善与电子转移(ET)速率的增强相关。原位X射线吸收近边缘结构分析表明,随着施加电势的降低, Ce 3+ /Ce 4+比率出现违反直觉的下降。这一发现表明 ET 从 Ce 3+转变为 O 2,并得到进一步电化学测量的支持。此外,原位拉曼光谱表明CNT参与了电催化。电化学测试和原位光谱学的结合提出了级联反应途径,其中O 2最初被Ce 3+还原,随后吸附到活性碳位点上。
更新日期:2024-04-19
中文翻译:

Ce4+/Ce3+ 氧化还原促进电子转移,用于双电子氧还原高效中性 H2O2 电合成
通过 2 电子氧还原反应 (2e – ORR)电化学生产 H 2 O 2提供了传统工业过程的清洁替代方案。非苛性中性电解质中的H 2 O 2电合成具有更广泛的应用前景;然而,与碱性电解质相比,它需要更大的过电势,即使使用不含金属的碳电催化剂也可以实现高 2e – ORR 活性。尽管二氧化铈已被广泛用作热催化和电催化反应中的催化促进剂,但其在增强中性 2e – ORR 中的作用很少被探索且仍不清楚。在这项工作中,我们制备了负载在碳纳米管(CeO x /CNT)复合催化剂上的二氧化铈纳米颗粒,并研究了二氧化铈对中性2e - ORR的促进作用。与单独的CNT相比,最佳的CeO x /CNT催化剂的ORR活性提高了1.5倍,并且H 2 O 2选择性超过87%。电化学阻抗谱表明活性的改善与电子转移(ET)速率的增强相关。原位X射线吸收近边缘结构分析表明,随着施加电势的降低, Ce 3+ /Ce 4+比率出现违反直觉的下降。这一发现表明 ET 从 Ce 3+转变为 O 2,并得到进一步电化学测量的支持。此外,原位拉曼光谱表明CNT参与了电催化。电化学测试和原位光谱学的结合提出了级联反应途径,其中O 2最初被Ce 3+还原,随后吸附到活性碳位点上。



























 京公网安备 11010802027423号
京公网安备 11010802027423号