当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
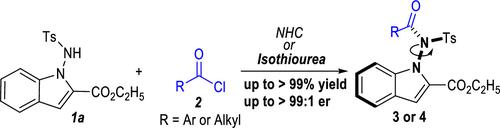
Catalytic N-Acylation for Access to N–N Atropisomeric N-Aminoindoles: Choice of Acylation Reagents and Mechanistic Insights
ACS Catalysis ( IF 12.9 ) Pub Date : 2024-04-18 , DOI: 10.1021/acscatal.4c00720 Chaoyang Song 1 , Chen Pang 1 , Youlin Deng 1 , Hui Cai 1 , Xiuhai Gan 1 , Yonggui Robin Chi 1, 2
ACS Catalysis ( IF 12.9 ) Pub Date : 2024-04-18 , DOI: 10.1021/acscatal.4c00720 Chaoyang Song 1 , Chen Pang 1 , Youlin Deng 1 , Hui Cai 1 , Xiuhai Gan 1 , Yonggui Robin Chi 1, 2
Affiliation
|
The synthesis of N–N axial compounds containing aromatic acyl amides using common acylation reagents remains challenging. We describe a highly atropenantioselective synthesis of N-aminoindoles containing N–N axes. A chiral cyclic isothiourea is used as the sole organic catalyst in the atropenantioselective transformation of the N-acylation reaction. Aroyl chlorides have been used as acylation reagents to construct atropisomeric compounds through N-acylation. The N-aminoindole products, which bear stereogenic N–N axes, were synthesized with high yields and enantioselectivities. Some of the enantiopure N-aminoindole products exhibited promising antibacterial activities against plant pathogens.
中文翻译:

催化 N-酰化获得 N-N 阻转异构 N-氨基吲哚:酰化试剂的选择和机理见解
使用常见酰化试剂合成含有芳香酰酰胺的 N-N 轴向化合物仍然具有挑战性。我们描述了含有 N-N 轴的 N-氨基吲哚的高度阿托潘对选择性合成。手性环状异硫脲用作 N-酰化反应的阿托苯对映选择性转化中的唯一有机催化剂。芳酰氯已被用作酰化试剂,通过 N-酰化构建阻转异构体化合物。 N-氨基吲哚产物具有立体 N-N 轴,具有高产率和对映选择性。一些对映体纯 N-氨基吲哚产品对植物病原体表现出良好的抗菌活性。
更新日期:2024-04-19
中文翻译:

催化 N-酰化获得 N-N 阻转异构 N-氨基吲哚:酰化试剂的选择和机理见解
使用常见酰化试剂合成含有芳香酰酰胺的 N-N 轴向化合物仍然具有挑战性。我们描述了含有 N-N 轴的 N-氨基吲哚的高度阿托潘对选择性合成。手性环状异硫脲用作 N-酰化反应的阿托苯对映选择性转化中的唯一有机催化剂。芳酰氯已被用作酰化试剂,通过 N-酰化构建阻转异构体化合物。 N-氨基吲哚产物具有立体 N-N 轴,具有高产率和对映选择性。一些对映体纯 N-氨基吲哚产品对植物病原体表现出良好的抗菌活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号