当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Conversion Reaction of the Zinc Sulfate Hydroxide Activated by Voltage Modulation for High-Performance Aqueous Zn/MnO2 Batteries
Advanced Energy Materials ( IF 27.8 ) Pub Date : 2024-04-17 , DOI: 10.1002/aenm.202303739 Zhen Wang 1 , Yixing Fang 1 , Jie Shi 1 , Zhihui Ma 1 , Xuanhui Qu 1 , Ping Li 1, 2
Advanced Energy Materials ( IF 27.8 ) Pub Date : 2024-04-17 , DOI: 10.1002/aenm.202303739 Zhen Wang 1 , Yixing Fang 1 , Jie Shi 1 , Zhihui Ma 1 , Xuanhui Qu 1 , Ping Li 1, 2
Affiliation
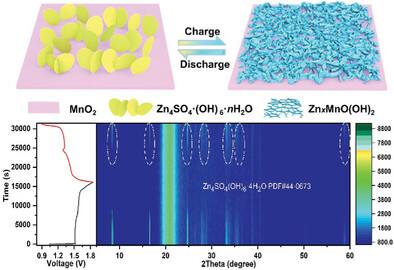
|
The reaction chemistry and degradation mechanism of MnO2-based cathodes remain controversial, which hinder their applications in energy storage. Herein, a conversion reaction between Zn4SO4·(OH)6·nH2O (ZSH) and ZnxMnO(OH)2 (ZMO) is proposed in ZnSO4-based electrolytes. The conversion reaction is an important component of the reaction chemistry as well as the Zn2+/H+ intercalation/deintercalation reaction, which not only provides electrochemical capacity but also dominates the degradation of MnO2 cathodes. The massive accumulation of inactive ZMO seriously destroying the dynamic performance of MnO2 cathodes is deemed to be a principal trigger of the degradation mechanism. Intriguingly, the conversion reaction is sensitive to voltage, which can be activated by voltage modulation. Active ZMO generated in the activated conversion reaction is endowed with higher reversibility and electrooxidation, avoiding the accumulation of inactive ZMO and the decline of kinetic performance, which are evidenced by MnO2 and ZSH cathodes. Accordingly, superior cycling stability with a capacity retention of 89.1% after 2000 cycles is achieved at 2 A g−1 for the MnO2 cathode equipped with the activated conversion reaction. Impressively, the conversion reaction activated by voltage modulation is applicable to various crystal forms of MnO2 (α-, β-, γ-, δ-), which is significant for the ZIBs with a long lifespan.
中文翻译:

调压激活的氢氧化硫酸锌转化反应用于高性能水系Zn/MnO2电池
MnO的反应化学及降解机理2 基阴极仍然存在争议,这阻碍了它们在储能方面的应用。在此,Zn之间的转化反应4 所以4 ·(哦)6 ·n H2 O (ZSH) 和 ZnX 2 (ZMO) 在 ZnSO 中被提出4 基电解质。转化反应与 Zn 一样,是反应化学的重要组成部分。2+ /H+ 嵌入/脱嵌反应,不仅提供电化学容量,而且主导MnO的降解2 阴极。非活性ZMO的大量积累严重破坏了MnO的动态性能2 阴极被认为是降解机制的主要触发因素。有趣的是,转化反应对电压敏感,可以通过电压调制来激活。活化转化反应中生成的活性ZMO具有较高的可逆性和电氧化性,避免了非活性ZMO的积累和动力学性能的下降,这在MnO中得到了证明2 和ZSH阴极。因此,在 2 A g-1 电流下,2000 次循环后容量保持率为 89.1%,具有出色的循环稳定性−1 对于二氧化锰2 配备有活化转化反应的阴极。令人印象深刻的是,通过电压调制激活的转化反应适用于各种晶型的MnO2 (α ‐,β ‐,γ ‐,δ ‐),这对于寿命较长的 ZIB 具有重要意义。
更新日期:2024-04-17
中文翻译:

调压激活的氢氧化硫酸锌转化反应用于高性能水系Zn/MnO2电池
MnO的反应化学及降解机理



























 京公网安备 11010802027423号
京公网安备 11010802027423号