当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Substituent-controlled divergent cyclization reactions of benzo[c][1,2]dithiol-3-ones and hexahydro-1,3,5-triazines
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2024-04-16 , DOI: 10.1039/d4qo00356j Bohao Zhang 1 , Sifan He 1 , Na Dong 1 , Antong Zhu 1 , Haojie Duan 1 , Dunjia Wang 1 , Yao Zhou 1
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2024-04-16 , DOI: 10.1039/d4qo00356j Bohao Zhang 1 , Sifan He 1 , Na Dong 1 , Antong Zhu 1 , Haojie Duan 1 , Dunjia Wang 1 , Yao Zhou 1
Affiliation
|
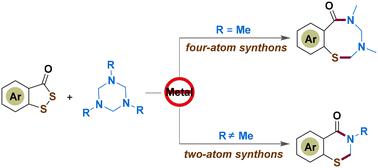
The first divergent cyclization reaction of benzo[c][1,2]dithiol-3-ones to assemble six- and eight-membered N-containing heterocycles is displayed herein. This unprecedented formal [4 + 4]/[4 + 2] cycloaddition of 3H-benzo[c][1,2]dithiol-3-ones and hexahydro-1,3,5-triazines is controlled by the N-substituent group of hexahydro-1,3,5-triazines. This newly discovered tactic features transition-metal-free, no inert gas protection, easy operation, mild conditions, decent yields, broad substrate scope and accessible scalability.
中文翻译:

苯并[c][1,2]二硫醇-3-酮和六氢-1,3,5-三嗪的取代基控制的发散环化反应
本文展示了苯并[ c ][1,2]二硫醇-3-酮组装六元和八元含氮杂环的第一个发散环化反应。 3 H-苯并[ c ][1,2]二硫醇-3-酮和六氢-1,3,5-三嗪的这种前所未有的正式[4 + 4]/[4 + 2]环加成反应由N-取代基控制六氢-1,3,5-三嗪基团。这种新发现的策略具有不含过渡金属、无惰性气体保护、操作简便、条件温和、收率良好、底物范围广泛和可扩展性等特点。
更新日期:2024-04-16
中文翻译:

苯并[c][1,2]二硫醇-3-酮和六氢-1,3,5-三嗪的取代基控制的发散环化反应
本文展示了苯并[ c ][1,2]二硫醇-3-酮组装六元和八元含氮杂环的第一个发散环化反应。 3 H-苯并[ c ][1,2]二硫醇-3-酮和六氢-1,3,5-三嗪的这种前所未有的正式[4 + 4]/[4 + 2]环加成反应由N-取代基控制六氢-1,3,5-三嗪基团。这种新发现的策略具有不含过渡金属、无惰性气体保护、操作简便、条件温和、收率良好、底物范围广泛和可扩展性等特点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号