当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In situ analysis of the oxygen evolution reaction on the CuO film in alkaline solution by surface interrogation scanning electrochemical microscopy: investigating active sites (CuIII) and kinetics
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2024-04-16 , DOI: 10.1039/d4ta00628c Seokjun Han 1 , Jinoh Yoo 1 , Won Tae Choi 1
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2024-04-16 , DOI: 10.1039/d4ta00628c Seokjun Han 1 , Jinoh Yoo 1 , Won Tae Choi 1
Affiliation
|
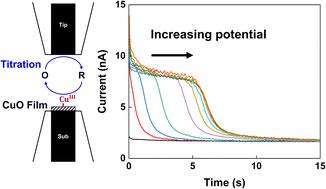
This study examines the CuO film as a non-precious metal catalyst for the oxygen evolution reaction (OER) in an alkaline solution (pH 13) using surface interrogation scanning electrochemical microscopy (SI-SECM). We identified a key potential of 0.72 V (vs. Ag/AgCl), at which the saturation of CuIII active sites on the CuO surface occurs (about 25 CuIII active sites per nm2), marking a significant increase in electrocatalytic OER activity. SI-SECM and Tafel slope analysis revealed varying concentrations of adsorbed intermediates depending on the applied potential during the OER. The combination of these findings with active site analysis led to the identification of the rate-determining step (RDS) for the OER on the CuO film. Furthermore, SI-SECM with controlled time-delay experiments was conducted to determine a rate constant of the reaction intermediates of 0.018 (±0.003) s−1. The results of this study highlight the potential of CuO as an effective and economical OER catalyst, offering critical insights for future development of CuO-based electrocatalysts.
中文翻译:

通过表面询问扫描电化学显微镜原位分析碱性溶液中 CuO 膜上的析氧反应:研究活性位点 (CuIII) 和动力学
本研究使用表面询问扫描电化学显微镜 (SI-SECM) 检查 CuO 薄膜作为非贵金属催化剂在碱性溶液 (pH 13) 中的析氧反应 (OER)。我们确定了 0.72 V 的关键电位(相对于Ag/AgCl),此时CuO 表面上的Cu III活性位点发生饱和(每 nm 2约 25 个 Cu III活性位点),标志着电催化 OER 活性显着增加。 SI-SECM 和 Tafel 斜率分析揭示了吸附中间体浓度的变化,具体取决于 OER 期间施加的电位。这些发现与活性位点分析相结合,确定了 CuO 薄膜上 OER 的速率决定步骤 (RDS)。此外,进行具有受控时间延迟实验的SI-SECM以确定0.018(±0.003)s -1的反应中间体的速率常数。这项研究的结果凸显了 CuO 作为有效且经济的 OER 催化剂的潜力,为 CuO 基电催化剂的未来发展提供了重要的见解。
更新日期:2024-04-16
中文翻译:

通过表面询问扫描电化学显微镜原位分析碱性溶液中 CuO 膜上的析氧反应:研究活性位点 (CuIII) 和动力学
本研究使用表面询问扫描电化学显微镜 (SI-SECM) 检查 CuO 薄膜作为非贵金属催化剂在碱性溶液 (pH 13) 中的析氧反应 (OER)。我们确定了 0.72 V 的关键电位(相对于Ag/AgCl),此时CuO 表面上的Cu III活性位点发生饱和(每 nm 2约 25 个 Cu III活性位点),标志着电催化 OER 活性显着增加。 SI-SECM 和 Tafel 斜率分析揭示了吸附中间体浓度的变化,具体取决于 OER 期间施加的电位。这些发现与活性位点分析相结合,确定了 CuO 薄膜上 OER 的速率决定步骤 (RDS)。此外,进行具有受控时间延迟实验的SI-SECM以确定0.018(±0.003)s -1的反应中间体的速率常数。这项研究的结果凸显了 CuO 作为有效且经济的 OER 催化剂的潜力,为 CuO 基电催化剂的未来发展提供了重要的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号