当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanism and Enantioselectivity in QUINOX-Catalyzed Asymmetric Allylations of Aromatic Aldehydes: Solvent and Substituent Effects
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2024-04-16 , DOI: 10.1021/acs.joc.4c00003 Pingli Lv 1, 2 , Rongxiu Zhu 2 , Dongju Zhang 2 , Steven E. Wheeler 3
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2024-04-16 , DOI: 10.1021/acs.joc.4c00003 Pingli Lv 1, 2 , Rongxiu Zhu 2 , Dongju Zhang 2 , Steven E. Wheeler 3
Affiliation
|
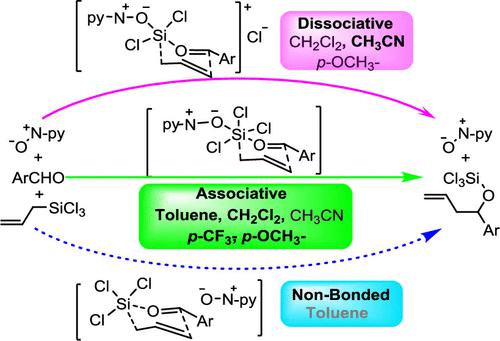
Computational investigations were conducted on the QUINOX-catalyzed asymmetric allylation of aromatic aldehydes with allyltrichlorosilanes. Our calculations provide evidence that the catalytic allylation can follow distinct mechanisms, depending on the solvent employed. In toluene and CH2Cl2, the QUINOX-catalyzed allylation predominantly follows an associative pathway, while in CH3CN, a dissociative pathway becomes more favorable. Noncovalent interactions, such as π-stacking effects for the associative mechanism and CH/π interactions for the dissociative mechanism, play a pivotal role in enantiostereodifferentiation in the asymmetric QUINOX-catalyzed reactions of benzaldehyde. Furthermore, the study unveils how different aldehyde substituents exert differing influences on the catalytic allylation reaction. Specifically, the QUINOX-catalyzed allylation of 4-(trifloromethyl)benzaldehyde displays a strong preference for the associative pathway, yielding excellent results in both yield and enantioselectivity. Conversely, 4-methoxybenzaldehyde tends to favor a dissociative mechanism with reduced yields and enantioselectivity. The mechanistic basis for these remarkable substituent effects on the catalytic allylation reaction was also elucidated. In summary, this research enhances our understanding of the QUINOX-catalyzed asymmetric allylation, shedding light on the role of solvents and substituents in the reaction mechanism and enantioselectivity.
中文翻译:

QUINOX 催化芳香醛不对称烯丙基化的机理和对映选择性:溶剂和取代基效应
对 QUINOX 催化的芳香醛与烯丙基三氯硅烷的不对称烯丙基化进行了计算研究。我们的计算提供了证据,表明催化烯丙基化可以遵循不同的机制,具体取决于所使用的溶剂。在甲苯和CH 2 Cl 2中,QUINOX催化的烯丙基化主要遵循缔合途径,而在CH 3 CN中,解离途径变得更有利。非共价相互作用,例如缔合机制的 π 堆积效应和解离机制的 CH/π 相互作用,在苯甲醛的不对称 QUINOX 催化反应中的对映体分化中发挥着关键作用。此外,该研究揭示了不同的醛取代基如何对催化烯丙基化反应产生不同的影响。具体而言,QUINOX 催化的 4-(三氟甲基)苯甲醛烯丙基化表现出对缔合途径的强烈偏好,在产率和对映选择性方面均产生了优异的结果。相反,4-甲氧基苯甲醛倾向于采用解离机制,但产率和对映选择性降低。还阐明了这些取代基对催化烯丙基化反应的显着影响的机制基础。总之,这项研究增强了我们对 QUINOX 催化的不对称烯丙基化的理解,揭示了溶剂和取代基在反应机理和对映选择性中的作用。
更新日期:2024-04-16
中文翻译:

QUINOX 催化芳香醛不对称烯丙基化的机理和对映选择性:溶剂和取代基效应
对 QUINOX 催化的芳香醛与烯丙基三氯硅烷的不对称烯丙基化进行了计算研究。我们的计算提供了证据,表明催化烯丙基化可以遵循不同的机制,具体取决于所使用的溶剂。在甲苯和CH 2 Cl 2中,QUINOX催化的烯丙基化主要遵循缔合途径,而在CH 3 CN中,解离途径变得更有利。非共价相互作用,例如缔合机制的 π 堆积效应和解离机制的 CH/π 相互作用,在苯甲醛的不对称 QUINOX 催化反应中的对映体分化中发挥着关键作用。此外,该研究揭示了不同的醛取代基如何对催化烯丙基化反应产生不同的影响。具体而言,QUINOX 催化的 4-(三氟甲基)苯甲醛烯丙基化表现出对缔合途径的强烈偏好,在产率和对映选择性方面均产生了优异的结果。相反,4-甲氧基苯甲醛倾向于采用解离机制,但产率和对映选择性降低。还阐明了这些取代基对催化烯丙基化反应的显着影响的机制基础。总之,这项研究增强了我们对 QUINOX 催化的不对称烯丙基化的理解,揭示了溶剂和取代基在反应机理和对映选择性中的作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号