当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Trivalent Phosphine-Catalyzed [4+1] Spiro-annulation Reaction Using Allenyl Imide and Methylene Cyclocompounds
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2024-04-16 , DOI: 10.1021/acs.joc.4c00388 Zi-Qiu Zhang 1 , Zhen-Kai Zhang 1 , Yu-Hao Wang 1 , Bo-Ting Chen 1 , Feng-Kai He 1 , Yi-Long Wang 1 , Tao Shu 1 , Yi-Yong Huang 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2024-04-16 , DOI: 10.1021/acs.joc.4c00388 Zi-Qiu Zhang 1 , Zhen-Kai Zhang 1 , Yu-Hao Wang 1 , Bo-Ting Chen 1 , Feng-Kai He 1 , Yi-Long Wang 1 , Tao Shu 1 , Yi-Yong Huang 1
Affiliation
|
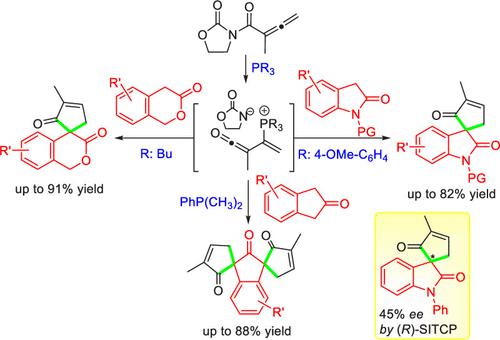
The trivalent phosphine-catalyzed [4+1] spiro-annulation reaction of allenyl imide and activated methylene cyclocompounds has been developed for the construction of various spiro-2-cyclopenten-1-ones. Oxindoles, 3-isochromanones, and 2-indanones are selected as 1C synthons to capture the in situ-generated bis-electrophilic α,β-unsaturated ketenyl phosphonium intermediate, affording the corresponding monospiro- and bispiro-cyclopentenones in good to excellent yields (≤91%) under mild conditions. The primary attempt at asymmetric catalysis using monophosphine (R)-SITCP provides promising enantioselectivity (45% ee). A plausible reaction mechanism is also proposed.
中文翻译:

三价膦催化烯酰亚胺和亚甲基环化合物的 [4+1] 螺环化反应
联烯基酰亚胺和活化的亚甲基环化合物的三价膦催化的[4+1]螺环化反应已被开发用于构建各种螺-2-环戊烯-1-酮。选择羟吲哚、3-异色满酮和 2-茚满酮作为 1C 合成子,捕获原位生成的双亲电子 α,β-不饱和烯基鏻中间体,以良好至优异的产率提供相应的单螺环和双螺环戊烯酮(≤ 91%)在温和条件下。使用单膦 ( R )-SITCP进行不对称催化的初步尝试提供了有希望的对映选择性 (45% ee)。还提出了一种合理的反应机制。
更新日期:2024-04-16
中文翻译:

三价膦催化烯酰亚胺和亚甲基环化合物的 [4+1] 螺环化反应
联烯基酰亚胺和活化的亚甲基环化合物的三价膦催化的[4+1]螺环化反应已被开发用于构建各种螺-2-环戊烯-1-酮。选择羟吲哚、3-异色满酮和 2-茚满酮作为 1C 合成子,捕获原位生成的双亲电子 α,β-不饱和烯基鏻中间体,以良好至优异的产率提供相应的单螺环和双螺环戊烯酮(≤ 91%)在温和条件下。使用单膦 ( R )-SITCP进行不对称催化的初步尝试提供了有希望的对映选择性 (45% ee)。还提出了一种合理的反应机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号