当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
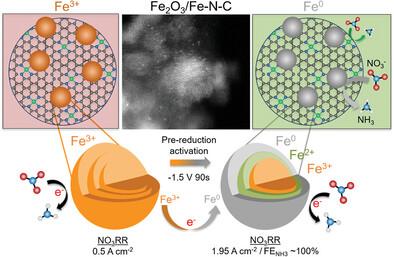
Synergizing Fe2O3 Nanoparticles on Single Atom Fe-N-C for Nitrate Reduction to Ammonia at Industrial Current Densities
Advanced Materials ( IF 29.4 ) Pub Date : 2024-04-15 , DOI: 10.1002/adma.202401133 Eamonn Murphy 1 , Baiyu Sun 1 , Martina Rüscher 2 , Yuanchao Liu 1 , Wenjie Zang 3 , Shengyuan Guo 1 , Yu‐Han Chen 1 , Uta Hejral 2 , Ying Huang 3 , Alvin Ly 3 , Iryna V. Zenyuk 1 , Xiaoqing Pan 3 , Janis Timoshenko 2 , Beatriz Roldán Cuenya 2 , Erik D. Spoerke 4 , Plamen Atanassov 1
Advanced Materials ( IF 29.4 ) Pub Date : 2024-04-15 , DOI: 10.1002/adma.202401133 Eamonn Murphy 1 , Baiyu Sun 1 , Martina Rüscher 2 , Yuanchao Liu 1 , Wenjie Zang 3 , Shengyuan Guo 1 , Yu‐Han Chen 1 , Uta Hejral 2 , Ying Huang 3 , Alvin Ly 3 , Iryna V. Zenyuk 1 , Xiaoqing Pan 3 , Janis Timoshenko 2 , Beatriz Roldán Cuenya 2 , Erik D. Spoerke 4 , Plamen Atanassov 1
Affiliation
|
The electrochemical reduction of nitrates (NO3−) enables a pathway for the carbon neutral synthesis of ammonia (NH3), via the nitrate reduction reaction (NO3RR), which has been demonstrated at high selectivity. However, to make NH3 synthesis cost-competitive with current technologies, high NH3 partial current densities (jNH3) must be achieved to reduce the levelized cost of NH3. Here, the high NO3RR activity of Fe-based materials is leveraged to synthesize a novel active particle-active support system with Fe2O3 nanoparticles supported on atomically dispersed Fe–N–C. The optimized 3×Fe2O3/Fe–N–C catalyst demonstrates an ultrahigh NO3RR activity, reaching a maximum jNH3 of 1.95 A cm−2 at a Faradaic efficiency (FE) for NH3 of 100% and an NH3 yield rate over 9 mmol hr−1 cm−2. Operando XANES and post-mortem XPS reveal the importance of a pre-reduction activation step, reducing the surface Fe2O3 (Fe3+) to highly active Fe0 sites, which are maintained during electrolysis. Durability studies demonstrate the robustness of both the Fe2O3 particles and Fe–Nx sites at highly cathodic potentials, maintaining a current of −1.3 A cm−2 over 24 hours. This work exhibits an effective and durable active particle-active support system enhancing the performance of the NO3RR, enabling industrially relevant current densities and near 100% selectivity.
中文翻译:

在单原子 Fe-N-C 上协同 Fe2O3 纳米粒子,在工业电流密度下将硝酸盐还原为氨
氧化氮物种的电化学还原为碳中性合成氨(NH3 )。氮的氧化程度最高的形式是硝酸盐 (NO3 - ) 可以还原为 NH3 通过电催化硝酸盐还原反应(NO3 RR),已被证明具有高选择性。然而,为了使NH3 与现有技术相比,合成成本具有竞争力,高 NH3 部分电流密度(j氨 )必须实现以降低NH的平准化成本3 。在这里,我们利用高NO3 铁基材料的 RR 活性合成新型活性颗粒活性载体系统2 氧3 纳米颗粒负载在原子分散的 Fe-N-C 上。通过协同纳米颗粒和单原子位点的活性,优化的 3xFe2 氧3 /Fe-N-C 催化剂表现出超高的 NO3 RR活动,达到最大j氨 1.95安厘米−2 NH 的法拉第效率 (FE)3 100% 和 NH3 产率超过 9 mmol hr−1 厘米−2 (相对于 RHE 为 ‐1.2 V)。原位 XANES 和死后 XPS 揭示了预还原活化步骤的重要性,减少了表面 Fe2 氧3 (铁3+ ) 到高活性 Fe0 电解过程中保留的位点,以实现超高NO3 RR 活动。耐久性研究证明了 Fe 的稳健性2 氧3 颗粒和Fe-NX 处于高阴极电位的位点,维持 ‐1.3 A cm 的电流−2 超过 24 小时,接近统一的 FE氨 (相对于 RHE 为 ‐1.0 V)。这项工作展示了一种有效且持久的活性颗粒活性支持系统,可增强 NO 的性能3 RR,实现工业相关的电流密度和接近 100% 的选择性。本文受版权保护。版权所有
更新日期:2024-04-15
中文翻译:

在单原子 Fe-N-C 上协同 Fe2O3 纳米粒子,在工业电流密度下将硝酸盐还原为氨
氧化氮物种的电化学还原为碳中性合成氨(NH



























 京公网安备 11010802027423号
京公网安备 11010802027423号