当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unraveling the Molecular Mechanism of H2O2 Production on Au–Pd Nanoalloy Surfaces
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2024-04-15 , DOI: 10.1021/acs.jpcc.4c00545 Wei Liu 1 , Liliang Tian 1 , Le Shi 1
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2024-04-15 , DOI: 10.1021/acs.jpcc.4c00545 Wei Liu 1 , Liliang Tian 1 , Le Shi 1
Affiliation
|
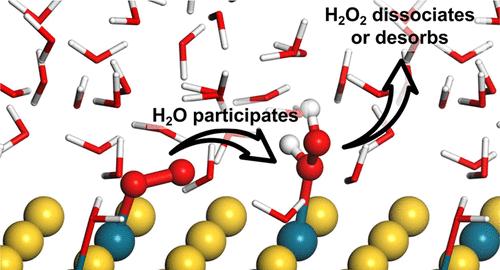
Oxygen reduction reaction (ORR) can proceed along two distinct pathways: the 4-electron pathway and the 2-electron pathway. The 4-electron pathway holds significant value in fuel cell technology, whereas the 2-electron pathway plays a crucial role in the industrial production of H2O2. Accurate prediction of the catalytic selectivity in the ORR stands as a pivotal factor in designing effective catalyst materials. It has been experimentally demonstrated that Au–Pd nanoalloy exhibit a high selectivity toward electrocatalytic H2O2 production. However, based on the widely employed computational hydrogen electrode method, the production of H2O on the surface of Au–Pd nanoalloy is more thermodynamically favorable, which shows a discrepancy with experimental results. In this work, we systematically investigate the influence of aqueous environment as well as electrode potential toward the ORR employing state-of-the-art ab initio molecular dynamics and metadynamics simulations. Our work reveals that the water molecules above the Au–Pd nanoalloy surface can alter the adsorption behavior of O2 and weaken the interaction between metal atom in the catalyst and oxygen atom in O2, therefore contributing to a high selectivity of Au–Pd nanoalloy toward H2O2 production. With a more negative electrode potential, the stability of H2O2 will decrease, and the corresponding selectivity will be lowered. These discoveries provide a dynamic perspective elucidating efficient H2O2 production on Au–Pd nanoalloy surfaces. Furthermore, they underscore the paramount significance of both the aqueous environment and electrode potential in shaping the ORR process.
中文翻译:

揭示 Au-Pd 纳米合金表面产生 H2O2 的分子机制
氧还原反应 (ORR) 可以沿着两种不同的途径进行:4 电子途径和 2 电子途径。 4电子途径在燃料电池技术中具有重要价值,而2电子途径在H 2 O 2的工业生产中起着至关重要的作用。准确预测 ORR 中的催化选择性是设计有效催化剂材料的关键因素。实验证明Au-Pd纳米合金对电催化H 2 O 2生产表现出高选择性。然而,基于广泛采用的计算氢电极方法,Au-Pd纳米合金表面H 2 O的产生在热力学上更为有利,这与实验结果存在差异。在这项工作中,我们采用最先进的从头算分子动力学和元动力学模拟,系统地研究了水环境以及电极电位对 ORR 的影响。我们的工作表明,Au-Pd纳米合金表面上方的水分子可以改变O 2的吸附行为,削弱催化剂中金属原子与O 2中氧原子之间的相互作用,从而有助于Au-Pd纳米合金的高选择性朝向H 2 O 2生产。电极电位越负,H 2 O 2的稳定性就会降低,相应的选择性也会降低。这些发现为阐明Au-Pd 纳米合金表面上H 2 O 2的高效生产提供了动态视角。此外,他们强调了水环境和电极电位在形成 ORR 过程中的至关重要性。
更新日期:2024-04-15
中文翻译:

揭示 Au-Pd 纳米合金表面产生 H2O2 的分子机制
氧还原反应 (ORR) 可以沿着两种不同的途径进行:4 电子途径和 2 电子途径。 4电子途径在燃料电池技术中具有重要价值,而2电子途径在H 2 O 2的工业生产中起着至关重要的作用。准确预测 ORR 中的催化选择性是设计有效催化剂材料的关键因素。实验证明Au-Pd纳米合金对电催化H 2 O 2生产表现出高选择性。然而,基于广泛采用的计算氢电极方法,Au-Pd纳米合金表面H 2 O的产生在热力学上更为有利,这与实验结果存在差异。在这项工作中,我们采用最先进的从头算分子动力学和元动力学模拟,系统地研究了水环境以及电极电位对 ORR 的影响。我们的工作表明,Au-Pd纳米合金表面上方的水分子可以改变O 2的吸附行为,削弱催化剂中金属原子与O 2中氧原子之间的相互作用,从而有助于Au-Pd纳米合金的高选择性朝向H 2 O 2生产。电极电位越负,H 2 O 2的稳定性就会降低,相应的选择性也会降低。这些发现为阐明Au-Pd 纳米合金表面上H 2 O 2的高效生产提供了动态视角。此外,他们强调了水环境和电极电位在形成 ORR 过程中的至关重要性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号