当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of Salt Concentration in Water‐In‐Salt Electrolyte on Supercapacitor Applications
ChemElectroChem ( IF 4 ) Pub Date : 2024-04-15 , DOI: 10.1002/celc.202400099 Imgon Hwang 1, 2 , Mantas Leketas 1, 2 , Kieran Griffiths 3 , Ryan Bragg 3 , John M. Griffin 3 , Robert A. W. Dryfe 1, 2
ChemElectroChem ( IF 4 ) Pub Date : 2024-04-15 , DOI: 10.1002/celc.202400099 Imgon Hwang 1, 2 , Mantas Leketas 1, 2 , Kieran Griffiths 3 , Ryan Bragg 3 , John M. Griffin 3 , Robert A. W. Dryfe 1, 2
Affiliation
|
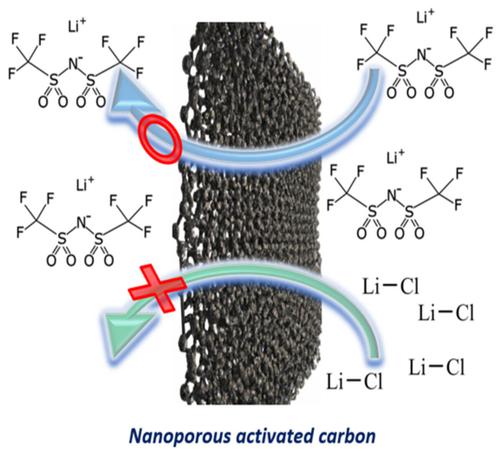
Electrical double‐layer supercapacitors offer numerous advantages in the context of energy storage; however, their widespread use is hindered by the high unit energy cost and low specific energy. Recently, water‐in‐salt (WIS) electrolytes have garnered interest for use in energy storage devices. Nevertheless, their direct application in high‐power devices is limited due to their high viscosity. In this study, we investigate the WIS Lithium bis(trifluoromethanesulfonyl)Imide (LiTFSI) electrolyte, revealing a high specific capacitance despite its elevated viscosity and restricted ionic conductivity. Our approach involves nuclear magnetic resonance (NMR) analysis alongside electrochemical analyses, highlighting the pronounced advantage of the WIS LiTFSI electrolyte over the WIS LiCl electrolyte at the molecular level. The NMR analysis shows that the LiTFSI electrolyte ions preferentially reside within the activated carbon pore network in the absence of an applied potential, in contrast to LiCl where the ions are more evenly distributed between the in‐pore and ex‐pore environments. This difference may contribute to the difference in capacitance between the two electrolytes observed during electrochemical cycling.
中文翻译:

盐包水电解质中盐浓度对超级电容器应用的影响
双层超级电容器在能量存储方面具有许多优势;然而,其广泛使用受到单位能源成本高和比能量低的阻碍。最近,盐包水(WIS)电解质在储能设备中的应用引起了人们的兴趣。然而,由于其高粘度,它们在高功率器件中的直接应用受到限制。在这项研究中,我们研究了 WIS 双(三氟甲磺酰)亚胺锂 (LiTFSI) 电解质,尽管其粘度较高且离子电导率有限,但仍具有较高的比电容。我们的方法涉及核磁共振 (NMR) 分析和电化学分析,突出了 WIS LiTFSI 电解质在分子水平上相对于 WIS LiCl 电解质的显着优势。 NMR 分析表明,在没有施加电势的情况下,LiTFSI 电解质离子优先驻留在活性炭孔隙网络内,而 LiCl 中的离子在孔内和孔外环境之间分布更均匀。这种差异可能导致电化学循环期间观察到的两种电解质之间的电容差异。
更新日期:2024-04-15
中文翻译:

盐包水电解质中盐浓度对超级电容器应用的影响
双层超级电容器在能量存储方面具有许多优势;然而,其广泛使用受到单位能源成本高和比能量低的阻碍。最近,盐包水(WIS)电解质在储能设备中的应用引起了人们的兴趣。然而,由于其高粘度,它们在高功率器件中的直接应用受到限制。在这项研究中,我们研究了 WIS 双(三氟甲磺酰)亚胺锂 (LiTFSI) 电解质,尽管其粘度较高且离子电导率有限,但仍具有较高的比电容。我们的方法涉及核磁共振 (NMR) 分析和电化学分析,突出了 WIS LiTFSI 电解质在分子水平上相对于 WIS LiCl 电解质的显着优势。 NMR 分析表明,在没有施加电势的情况下,LiTFSI 电解质离子优先驻留在活性炭孔隙网络内,而 LiCl 中的离子在孔内和孔外环境之间分布更均匀。这种差异可能导致电化学循环期间观察到的两种电解质之间的电容差异。



























 京公网安备 11010802027423号
京公网安备 11010802027423号