当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of actin dimerization inducers inspired by actin-depolymerizing macrolides
Chemical Communications ( IF 4.9 ) Pub Date : 2024-04-15 , DOI: 10.1039/d4cc01304b Moeka Itakura 1 , Didik Huswo Utomo 1, 2 , Masaki Kita 1
Chemical Communications ( IF 4.9 ) Pub Date : 2024-04-15 , DOI: 10.1039/d4cc01304b Moeka Itakura 1 , Didik Huswo Utomo 1, 2 , Masaki Kita 1
Affiliation
|
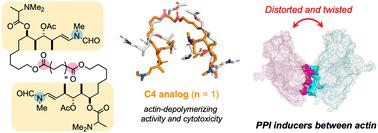
Several natural cytotoxic C2-symmetric bis-lactones, such as swinholide A and rhizopodin, sequester actin dimer from the actin network and potently inhibit actin dynamics. To develop new protein–protein interaction (PPI) modulators, we synthesized structurally simplified actin-binding side-chain dimers of antitumor macrolide aplyronine A. By fixing the two side-chains closer than those of rhizopodin, the C4 linker analog depolymerized filamentous actin more potently than natural aplyronines. Cross-link experiments revealed that actin dimer was formed by treatment with the C4 linker analog. Molecular dynamics simulations showed that this analog significantly changed the interaction and spatial arrangement of the two actins compared to those in rhizopodin to provide a highly distorted and twisted orientation in the complex. Our study may promote the development of PPI-based anticancer and other drug leads related to cytoskeletal dynamics.
中文翻译:

受肌动蛋白解聚大环内酯启发开发肌动蛋白二聚化诱导剂
几种天然细胞毒性C 2 -对称双内酯,例如 swinholide A 和 rhizopodin,可将肌动蛋白二聚体与肌动蛋白网络隔离,并有效抑制肌动蛋白动力学。为了开发新的蛋白质-蛋白质相互作用 (PPI) 调节剂,我们合成了结构简化的抗肿瘤大环内酯 aplyronine A 的肌动蛋白结合侧链二聚体。通过将两个侧链固定得比根足素更近,C4 接头类似物可以更好地解聚丝状肌动蛋白。比天然 aplyronines 更有效。交联实验表明,肌动蛋白二聚体是通过 C4 连接子类似物处理形成的。分子动力学模拟表明,与根足蛋白相比,这种类似物显着改变了两种肌动蛋白的相互作用和空间排列,从而在复合物中提供了高度扭曲和扭曲的方向。我们的研究可能会促进基于 PPI 的抗癌药物和其他与细胞骨架动力学相关的先导药物的开发。
更新日期:2024-04-16
中文翻译:

受肌动蛋白解聚大环内酯启发开发肌动蛋白二聚化诱导剂
几种天然细胞毒性C 2 -对称双内酯,例如 swinholide A 和 rhizopodin,可将肌动蛋白二聚体与肌动蛋白网络隔离,并有效抑制肌动蛋白动力学。为了开发新的蛋白质-蛋白质相互作用 (PPI) 调节剂,我们合成了结构简化的抗肿瘤大环内酯 aplyronine A 的肌动蛋白结合侧链二聚体。通过将两个侧链固定得比根足素更近,C4 接头类似物可以更好地解聚丝状肌动蛋白。比天然 aplyronines 更有效。交联实验表明,肌动蛋白二聚体是通过 C4 连接子类似物处理形成的。分子动力学模拟表明,与根足蛋白相比,这种类似物显着改变了两种肌动蛋白的相互作用和空间排列,从而在复合物中提供了高度扭曲和扭曲的方向。我们的研究可能会促进基于 PPI 的抗癌药物和其他与细胞骨架动力学相关的先导药物的开发。



























 京公网安备 11010802027423号
京公网安备 11010802027423号