当前位置:
X-MOL 学术
›
CNS Neurosci. Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nuclear autoantigenic sperm protein facilitates glioblastoma progression and radioresistance by regulating the ANXA2/STAT3 axis
CNS Neuroscience & Therapeutics ( IF 5.5 ) Pub Date : 2024-04-12 , DOI: 10.1111/cns.14709 Yuning Qiu 1, 2 , Dongling Pei 1 , Minkai Wang 1 , Qimeng Wang 2, 3 , Wenchao Duan 1 , Li Wang 3 , Kehan Liu 2, 3 , Yu Guo 1 , Lin Luo 1 , Zhixuan Guo 1 , Fangzhan Guan 1 , Zilong Wang 1 , Aoqi Xing 1 , Zhongyi Liu 1 , Zeyu Ma 1 , Guozhong Jiang 3 , Dongming Yan 1 , Xianzhi Liu 1 , Zhenyu Zhang 1 , Weiwei Wang 3
CNS Neuroscience & Therapeutics ( IF 5.5 ) Pub Date : 2024-04-12 , DOI: 10.1111/cns.14709 Yuning Qiu 1, 2 , Dongling Pei 1 , Minkai Wang 1 , Qimeng Wang 2, 3 , Wenchao Duan 1 , Li Wang 3 , Kehan Liu 2, 3 , Yu Guo 1 , Lin Luo 1 , Zhixuan Guo 1 , Fangzhan Guan 1 , Zilong Wang 1 , Aoqi Xing 1 , Zhongyi Liu 1 , Zeyu Ma 1 , Guozhong Jiang 3 , Dongming Yan 1 , Xianzhi Liu 1 , Zhenyu Zhang 1 , Weiwei Wang 3
Affiliation
|
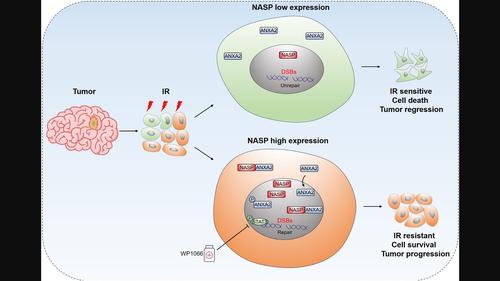
AimsAlthough radiotherapy is a core treatment modality for various human cancers, including glioblastoma multiforme (GBM), its clinical effects are often limited by radioresistance. The specific molecular mechanisms underlying radioresistance are largely unknown, and the reduction of radioresistance is an unresolved challenge in GBM research.MethodsWe analyzed and verified the expression of nuclear autoantigenic sperm protein (NASP) in gliomas and its relationship with patient prognosis. We also explored the function of NASP in GBM cell lines. We performed further mechanistic experiments to investigate the mechanisms by which NASP facilitates GBM progression and radioresistance. An intracranial mouse model was used to verify the effectiveness of combination therapy.ResultsNASP was highly expressed in gliomas, and its expression was negatively correlated with the prognosis of glioma. Functionally, NASP facilitated GBM cell proliferation, migration, invasion, and radioresistance. Mechanistically, NASP interacted directly with annexin A2 (ANXA2) and promoted its nuclear localization, which may have been mediated by phospho‐annexin A2 (Tyr23). The NASP/ANXA2 axis was involved in DNA damage repair after radiotherapy, which explains the radioresistance of GBM cells that highly express NASP. NASP overexpression significantly activated the signal transducer and activator of transcription 3 (STAT3) signaling pathway. The combination of WP1066 (a STAT3 pathway inhibitor) and radiotherapy significantly inhibited GBM growth in vitro and in vivo.ConclusionOur findings indicate that NASP may serve as a potential biomarker of GBM radioresistance and has important implications for improving clinical radiotherapy.
中文翻译:

核自身抗原精子蛋白通过调节 ANXA2/STAT3 轴促进胶质母细胞瘤进展和放射抗性
目标虽然放射治疗是包括多形性胶质母细胞瘤(GBM)在内的多种人类癌症的核心治疗方式,但其临床效果往往受到放射抗性的限制。放射抵抗的具体分子机制尚不清楚,放射抵抗的降低是GBM研究中尚未解决的挑战。方法我们分析并验证了胶质瘤中核自身抗原精子蛋白(NASP)的表达及其与患者预后的关系。我们还探讨了 NASP 在 GBM 细胞系中的功能。我们进行了进一步的机制实验来研究 NASP 促进 GBM 进展和放射抗性的机制。采用颅内小鼠模型验证联合治疗的有效性。结果NASP在胶质瘤中高表达,其表达与胶质瘤的预后呈负相关。从功能上讲,NASP 促进 GBM 细胞增殖、迁移、侵袭和放射抗性。从机制上讲,NASP 直接与膜联蛋白 A2 (ANXA2) 相互作用并促进其核定位,这可能是由磷酸膜联蛋白 A2 (Tyr23) 介导的。 NASP/ANXA2轴参与放射治疗后的DNA损伤修复,这解释了高表达NASP的GBM细胞的放射抗性。 NASP 过表达显着激活信号转导子和转录激活子 3 (STAT3) 信号通路。 WP1066(一种 STAT3 通路抑制剂)与放疗的组合在体外和体内显着抑制 GBM 生长。结论我们的研究结果表明 NASP 可能作为 GBM 放射抗性的潜在生物标志物,对改善临床放疗具有重要意义。
更新日期:2024-04-12
中文翻译:

核自身抗原精子蛋白通过调节 ANXA2/STAT3 轴促进胶质母细胞瘤进展和放射抗性
目标虽然放射治疗是包括多形性胶质母细胞瘤(GBM)在内的多种人类癌症的核心治疗方式,但其临床效果往往受到放射抗性的限制。放射抵抗的具体分子机制尚不清楚,放射抵抗的降低是GBM研究中尚未解决的挑战。方法我们分析并验证了胶质瘤中核自身抗原精子蛋白(NASP)的表达及其与患者预后的关系。我们还探讨了 NASP 在 GBM 细胞系中的功能。我们进行了进一步的机制实验来研究 NASP 促进 GBM 进展和放射抗性的机制。采用颅内小鼠模型验证联合治疗的有效性。结果NASP在胶质瘤中高表达,其表达与胶质瘤的预后呈负相关。从功能上讲,NASP 促进 GBM 细胞增殖、迁移、侵袭和放射抗性。从机制上讲,NASP 直接与膜联蛋白 A2 (ANXA2) 相互作用并促进其核定位,这可能是由磷酸膜联蛋白 A2 (Tyr23) 介导的。 NASP/ANXA2轴参与放射治疗后的DNA损伤修复,这解释了高表达NASP的GBM细胞的放射抗性。 NASP 过表达显着激活信号转导子和转录激活子 3 (STAT3) 信号通路。 WP1066(一种 STAT3 通路抑制剂)与放疗的组合在体外和体内显着抑制 GBM 生长。结论我们的研究结果表明 NASP 可能作为 GBM 放射抗性的潜在生物标志物,对改善临床放疗具有重要意义。



























 京公网安备 11010802027423号
京公网安备 11010802027423号