当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
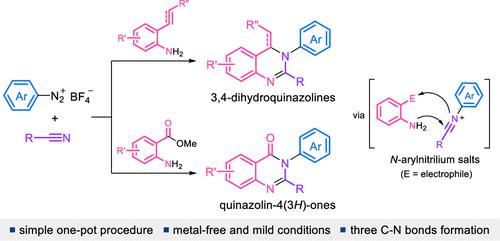
One-Pot Three-Component Reaction for the Synthesis of 3,4-Dihydroquinazolines and Quinazolin-4(3H)-ones
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2024-04-12 , DOI: 10.1021/acs.joc.4c00458 Shiwei Chen 1 , Yeong Shin Ji 1 , Yuri Choi 1 , So Won Youn 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2024-04-12 , DOI: 10.1021/acs.joc.4c00458 Shiwei Chen 1 , Yeong Shin Ji 1 , Yuri Choi 1 , So Won Youn 1
Affiliation
|
A highly efficient and straightforward one-pot synthesis of diversely substituted 3,4-dihydroquinazolines and quinazolin-4(3H)-ones has been achieved through a domino three-component assembly reaction of arenediazonium salts, nitriles, and bifunctional aniline derivatives. This new protocol involves three C–N bond formations through the initial formation of N-arylnitrilium intermediates from arenediazonium salts and nitriles, followed by the sequential nucleophilic addition and cyclization reactions with bifunctional anilines, leading to such N-heterocyclic compounds of biological and pharmacological importance. This method offers a simple, expedient, and robust approach with the use of amenable and easily accessible reactants/reagents under metal-free mild conditions, good functional group tolerance, and high efficiency. The synthetic applications were also demonstrated by derivatization of the products obtained from these processes and syntheses of a diverse range of valuable polycyclic N-heterocycles.
中文翻译:

一锅三组分反应合成3,4-二氢喹唑啉和喹唑啉-4(3H)-酮
通过芳烃重氮盐、腈和双官能苯胺衍生物的多米诺三组分组装反应,实现了多种取代的3,4-二氢喹唑啉和喹唑啉-4(3 H )-酮的高效、简单的一锅合成。这个新方案涉及三个C-N键的形成,首先由芳烃重氮盐和腈形成N-芳基腈中间体,然后与双功能苯胺进行连续的亲核加成和环化反应,从而产生具有生物和药理学重要性的N-杂环化合物。该方法提供了一种简单、方便、稳健的方法,在无金属的温和条件下使用易于使用且易于获得的反应物/试剂,具有良好的官能团耐受性和高效率。合成应用还通过从这些过程获得的产物的衍生化和各种有价值的多环N-杂环的合成来证明。
更新日期:2024-04-12
中文翻译:

一锅三组分反应合成3,4-二氢喹唑啉和喹唑啉-4(3H)-酮
通过芳烃重氮盐、腈和双官能苯胺衍生物的多米诺三组分组装反应,实现了多种取代的3,4-二氢喹唑啉和喹唑啉-4(3 H )-酮的高效、简单的一锅合成。这个新方案涉及三个C-N键的形成,首先由芳烃重氮盐和腈形成N-芳基腈中间体,然后与双功能苯胺进行连续的亲核加成和环化反应,从而产生具有生物和药理学重要性的N-杂环化合物。该方法提供了一种简单、方便、稳健的方法,在无金属的温和条件下使用易于使用且易于获得的反应物/试剂,具有良好的官能团耐受性和高效率。合成应用还通过从这些过程获得的产物的衍生化和各种有价值的多环N-杂环的合成来证明。



























 京公网安备 11010802027423号
京公网安备 11010802027423号