当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nickel-Catalyzed Deoxygenative Disulfuration of Alcohols to Access Unsymmetrical Disulfides
ACS Catalysis ( IF 12.9 ) Pub Date : 2024-04-11 , DOI: 10.1021/acscatal.4c00814 Xiyao Chen 1 , Wen Shao 1 , Guo-Jun Deng 1, 2
ACS Catalysis ( IF 12.9 ) Pub Date : 2024-04-11 , DOI: 10.1021/acscatal.4c00814 Xiyao Chen 1 , Wen Shao 1 , Guo-Jun Deng 1, 2
Affiliation
|
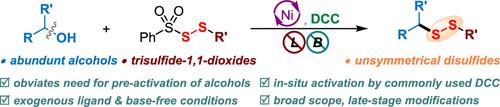
Given the abundance of alcohol feedstocks and the significance of disulfides, we herein report a nickel-catalyzed direct deoxygenative disulfuration of alcohols with trisulfide dioxides to access a wide range of disulfide molecules without the cumbersome decoration of coupling partners. The use of readily available dicyclohexylcarbodiimide to form transient isoureas provides the activation of the high bond dissociation energy of the C–O bond, which facilitates the straightforward conversion of nonderivatized alcohols to forge a C–SS bond. Notably, this method obviates a preactivation multistep procedure and provides a catalytic turnover under exogenous ligand and base-free conditions, featuring a broad substrate scope and functional group compatibility. It thus offers a robust alternative to existing methods for the precise construction of versatile disulfide compounds from more abundant and commercially available substrates. The synthetic utility of the method was further showcased by successful gram-scale experiments and disulfuration of structurally complex pharmaceuticals.
中文翻译:

镍催化醇脱氧二硫化得到不对称二硫化物
考虑到醇原料的丰富性和二硫化物的重要性,我们在此报告了一种镍催化的醇与三硫化物二氧化物的直接脱氧二硫化反应,以获得广泛的二硫化物分子,而无需繁琐的偶联配偶体修饰。使用容易获得的二环己基碳二亚胺形成瞬态异脲可以激活C-O键的高键解离能,从而促进非衍生化醇直接转化为C-SS键。值得注意的是,该方法避免了预激活多步骤程序,并在外源配体和无碱条件下提供催化转换,具有广泛的底物范围和官能团兼容性。因此,它为现有方法提供了一种强大的替代方法,可以从更丰富且市售的底物中精确构建多功能二硫化合物。通过成功的克级实验和结构复杂药物的二硫化,进一步展示了该方法的合成效用。
更新日期:2024-04-13
中文翻译:

镍催化醇脱氧二硫化得到不对称二硫化物
考虑到醇原料的丰富性和二硫化物的重要性,我们在此报告了一种镍催化的醇与三硫化物二氧化物的直接脱氧二硫化反应,以获得广泛的二硫化物分子,而无需繁琐的偶联配偶体修饰。使用容易获得的二环己基碳二亚胺形成瞬态异脲可以激活C-O键的高键解离能,从而促进非衍生化醇直接转化为C-SS键。值得注意的是,该方法避免了预激活多步骤程序,并在外源配体和无碱条件下提供催化转换,具有广泛的底物范围和官能团兼容性。因此,它为现有方法提供了一种强大的替代方法,可以从更丰富且市售的底物中精确构建多功能二硫化合物。通过成功的克级实验和结构复杂药物的二硫化,进一步展示了该方法的合成效用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号