Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Palladium‐Catalyzed Atroposelective Suzuki–Miyaura Coupling to Construct Axially Chiral Tetra‐Substituted α‐Boryl Styrenes
Advanced Science ( IF 15.1 ) Pub Date : 2024-04-11 , DOI: 10.1002/advs.202309706 Xiaorui Li 1 , Lingyu Kong 1 , Shuxin Yin 1 , Hengrui Zhou 1 , Aijun Lin 1 , Hequan Yao 1 , Shang Gao 1
Advanced Science ( IF 15.1 ) Pub Date : 2024-04-11 , DOI: 10.1002/advs.202309706 Xiaorui Li 1 , Lingyu Kong 1 , Shuxin Yin 1 , Hengrui Zhou 1 , Aijun Lin 1 , Hequan Yao 1 , Shang Gao 1
Affiliation
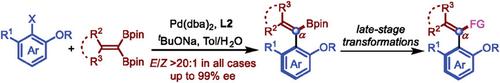
|
Palladium‐catalyzed Suzuki–Miyaura (SM) coupling is a valuable method for forming C─C bonds, including those between aryl moieties. However, achieving atroposelective synthesis of axially chiral styrenes via SM coupling remains challenging. In this study, a palladium‐catalyzed atroposelective Suzuki–Miyaura coupling between gem ‐diborylalkenes and aryl halides is presented. Using the monophosphine ligand Me‐BI‐DIME (L2), a range of axially chiral tetra‐substituted acyclic styrenes with high yields and excellent enantioselectivities are successfully synthesized. Control experiments reveal that the gem ‐diboryl group significantly influences the product enantioselectivities and the coupling prefers to occur at sites with lower steric hindrance. Additionally, the alkenyl boronate group in the products proves versatile, allowing for various transformations while maintaining high optical purities.
中文翻译:

钯催化天体选择性 Suzuki-Miyaura 偶联构建轴向手性四取代 α-硼基苯乙烯
钯催化的铃木-宫浦 (SM) 偶联是形成 C─C 键(包括芳基部分之间的键)的一种有价值的方法。然而,通过 SM 偶联实现轴向手性苯乙烯的天体选择性合成仍然具有挑战性。在这项研究中,钯催化的铃木-宫浦耦合宝石 提出了-二硼基烯烃和芳基卤化物。使用单膦配体 Me-BI-DIME (L2),成功合成了一系列具有高产率和优异对映选择性的轴向手性四取代无环苯乙烯。对照实验表明宝石 ‐二硼基显着影响产物的对映选择性,并且偶联更倾向于发生在空间位阻较低的位点。此外,产品中的烯基硼酸酯基团具有多种用途,可以在保持高光学纯度的同时进行各种转化。
更新日期:2024-04-11
中文翻译:

钯催化天体选择性 Suzuki-Miyaura 偶联构建轴向手性四取代 α-硼基苯乙烯
钯催化的铃木-宫浦 (SM) 偶联是形成 C─C 键(包括芳基部分之间的键)的一种有价值的方法。然而,通过 SM 偶联实现轴向手性苯乙烯的天体选择性合成仍然具有挑战性。在这项研究中,钯催化的铃木-宫浦耦合



























 京公网安备 11010802027423号
京公网安备 11010802027423号