当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Discovery of a new class of potent pyrrolo[3,4-c]quinoline-1,3-diones based inhibitors of human dihydroorotate dehydrogenase: Synthesis, pharmacological and toxicological evaluation
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2024-04-09 , DOI: 10.1016/j.bioorg.2024.107359 Marina G. Dimitrijević , Cornelia Roschger , Kevin Lang , Andreas Zierer , Milica G. Paunović , Ana D. Obradović , Miloš M. Matić , Marijana Pocrnić , Nives Galić , Andrija Ćirić , Milan D. Joksović
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2024-04-09 , DOI: 10.1016/j.bioorg.2024.107359 Marina G. Dimitrijević , Cornelia Roschger , Kevin Lang , Andreas Zierer , Milica G. Paunović , Ana D. Obradović , Miloš M. Matić , Marijana Pocrnić , Nives Galić , Andrija Ćirić , Milan D. Joksović
|
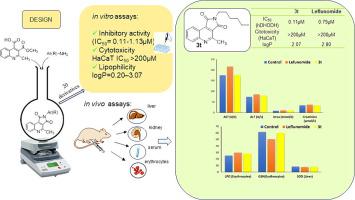
Twenty -substituted pyrrolo[3,4-]quinoline-1,3-diones were synthesized by a cyclization reaction of Pfitzinger's quinoline ester precursor with the selected aromatic, heteroaromatic and aliphatic amines. The structures of all derivatives were confirmed by IR, H NMR, C NMR and HRMS spectra, while their purity was determined using HPLC techniques. Almost all compounds were identified as a new class ofpotent inhibitors against hDHODH among which and were the most active ones with the same IC values of 0.11 μM, about seven times better than reference drug leflunomide. These two derivatives also exhibited very low cytotoxic effects toward healthy HaCaT cells and the optimal lipophilic properties with logP value of 1.12 and 2.07 respectively, obtained experimentally at physiological pH. We further evaluated the comparative differences in toxicological impact of the three most active compounds , and and reference drug leflunomide. The rats were divided into five groups and were treated intraperitoneally, control group (group I) with a single dose of leflunomide (20 mg/kg) group II and the other three groups, III, IV and V were treated with , and (20 mg/kg bw) separately. The investigation was performed in liver, kidney and blood by examining serum biochemical parameters and parameters of oxidative stress.
中文翻译:

发现一类新的有效吡咯并[3,4-c]喹啉-1,3-二酮类人二氢乳清酸脱氢酶抑制剂:合成、药理学和毒理学评价
通过 Pfitzinger 喹啉酯前体与选定的芳香胺、杂芳香胺和脂肪胺的环化反应合成了二十个取代的吡咯并[3,4-]喹啉-1,3-二酮。所有衍生物的结构均通过IR、H NMR、C NMR 和HRMS 光谱进行确认,同时使用HPLC 技术测定其纯度。几乎所有化合物都被鉴定为一类新的强效 hDHODH 抑制剂,其中 和 是活性最强的化合物,其 IC 值为 0.11 μM,比参考药物来氟米特好约 7 倍。这两种衍生物还对健康 HaCaT 细胞表现出非常低的细胞毒性作用,并在生理 pH 下通过实验获得了最佳的亲脂性,logP 值分别为 1.12 和 2.07。我们进一步评估了三种最活跃的化合物和参考药物来氟米特的毒理学影响的比较差异。将大鼠分为5组,腹腔注射治疗,对照组(I组)单剂量来氟米特(20 mg/kg),II组,其余三组(III、IV、V)腹腔注射,(20毫克/千克体重)单独。通过检查血清生化参数和氧化应激参数,在肝脏、肾脏和血液中进行了研究。
更新日期:2024-04-09
中文翻译:

发现一类新的有效吡咯并[3,4-c]喹啉-1,3-二酮类人二氢乳清酸脱氢酶抑制剂:合成、药理学和毒理学评价
通过 Pfitzinger 喹啉酯前体与选定的芳香胺、杂芳香胺和脂肪胺的环化反应合成了二十个取代的吡咯并[3,4-]喹啉-1,3-二酮。所有衍生物的结构均通过IR、H NMR、C NMR 和HRMS 光谱进行确认,同时使用HPLC 技术测定其纯度。几乎所有化合物都被鉴定为一类新的强效 hDHODH 抑制剂,其中 和 是活性最强的化合物,其 IC 值为 0.11 μM,比参考药物来氟米特好约 7 倍。这两种衍生物还对健康 HaCaT 细胞表现出非常低的细胞毒性作用,并在生理 pH 下通过实验获得了最佳的亲脂性,logP 值分别为 1.12 和 2.07。我们进一步评估了三种最活跃的化合物和参考药物来氟米特的毒理学影响的比较差异。将大鼠分为5组,腹腔注射治疗,对照组(I组)单剂量来氟米特(20 mg/kg),II组,其余三组(III、IV、V)腹腔注射,(20毫克/千克体重)单独。通过检查血清生化参数和氧化应激参数,在肝脏、肾脏和血液中进行了研究。



























 京公网安备 11010802027423号
京公网安备 11010802027423号