当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, selective synthesis and biological activities evaluation of novel thiazol-2-ylbenzamide and thiazole-2-ylbenzimidoyl chloride derivatives
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2024-04-06 , DOI: 10.1016/j.bioorg.2024.107333 Zonghan Xu , Xiang Cheng , Hongyun Cui , Linmin Cao , Yaping Song , Xihao Chang , Dandan Wang , Xianhai Lv
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2024-04-06 , DOI: 10.1016/j.bioorg.2024.107333 Zonghan Xu , Xiang Cheng , Hongyun Cui , Linmin Cao , Yaping Song , Xihao Chang , Dandan Wang , Xianhai Lv
|
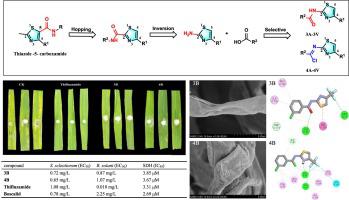
To promote the development and exploitation of novel antifungal agents, a series of thiazol-2-ylbenzamide derivatives (-) and thiazole-2-ylbenzimidoyl chloride derivatives (-) were designed and selective synthesis. The bioassay results showed that most of the target compounds exhibited excellent antifungal activities against five plant pathogenic fungi (, , , and ). The antifungal effects of compounds (EC = 0.72 mg/L) and (EC = 0.65 mg/L) against were comparable to succinate dehydrogenase inhibitors (SDHIs) (EC = 1.08 mg/L) and (EC = 0.78 mg/L). Especially, compounds (EC = 0.87 mg/L) and (EC = 1.08 mg/L) showed higher activity against than (EC = 2.25 mg/L). experiments in rice leaves revealed that compounds (86.8 %) and (85.3 %) exhibited excellent protective activities against comparable to (88.5 %). Scanning electron microscopy (SEM) results exhibited that compounds and dramatically disrupted the typical structure and morphology of mycelium. Molecular docking demonstrated that compounds and had significant interactions with succinate dehydrogenase (SDH). Meanwhile, SDH inhibition assay results further proved their potential as SDHIs. In addition, acute oral toxicity tests on L. showed only low toxicity for compounds and to L. populations. These results suggested that these two series of compounds had merit for further investigation as potential low-risk agricultural SDHI fungicides.
中文翻译:

新型噻唑-2-基苯甲酰胺和噻唑-2-基苯甲酰氯衍生物的设计、选择性合成及生物活性评价
为了促进新型抗真菌药物的开发利用,设计并选择性合成了一系列噻唑-2-基苯甲酰胺衍生物(-)和噻唑-2-基苯甲酰氯衍生物(-)。生物测定结果表明,大部分目标化合物对5种植物病原真菌(、、、和)表现出优异的抗真菌活性。化合物(EC = 0.72 mg/L)和(EC = 0.65 mg/L)的抗真菌作用与琥珀酸脱氢酶抑制剂(SDHI)(EC = 1.08 mg/L)和(EC = 0.78 mg/L)相当。特别是,化合物(EC = 0.87 mg/L)和(EC = 1.08 mg/L)表现出比(EC = 2.25 mg/L)更高的活性。稻叶实验表明,化合物(86.8%)和(85.3%)表现出与(88.5%)相当的优异保护活性。扫描电子显微镜(SEM)结果表明,该化合物极大地破坏了菌丝体的典型结构和形态。分子对接表明化合物 和 与琥珀酸脱氢酶 (SDH) 具有显着的相互作用。同时,SDH抑制实验结果进一步证明了它们作为SDHI的潜力。此外,对 L. 的急性口服毒性测试显示,化合物和 L. 群体的毒性较低。这些结果表明这两个系列的化合物作为潜在的低风险农用 SDHI 杀菌剂值得进一步研究。
更新日期:2024-04-06
中文翻译:

新型噻唑-2-基苯甲酰胺和噻唑-2-基苯甲酰氯衍生物的设计、选择性合成及生物活性评价
为了促进新型抗真菌药物的开发利用,设计并选择性合成了一系列噻唑-2-基苯甲酰胺衍生物(-)和噻唑-2-基苯甲酰氯衍生物(-)。生物测定结果表明,大部分目标化合物对5种植物病原真菌(、、、和)表现出优异的抗真菌活性。化合物(EC = 0.72 mg/L)和(EC = 0.65 mg/L)的抗真菌作用与琥珀酸脱氢酶抑制剂(SDHI)(EC = 1.08 mg/L)和(EC = 0.78 mg/L)相当。特别是,化合物(EC = 0.87 mg/L)和(EC = 1.08 mg/L)表现出比(EC = 2.25 mg/L)更高的活性。稻叶实验表明,化合物(86.8%)和(85.3%)表现出与(88.5%)相当的优异保护活性。扫描电子显微镜(SEM)结果表明,该化合物极大地破坏了菌丝体的典型结构和形态。分子对接表明化合物 和 与琥珀酸脱氢酶 (SDH) 具有显着的相互作用。同时,SDH抑制实验结果进一步证明了它们作为SDHI的潜力。此外,对 L. 的急性口服毒性测试显示,化合物和 L. 群体的毒性较低。这些结果表明这两个系列的化合物作为潜在的低风险农用 SDHI 杀菌剂值得进一步研究。



























 京公网安备 11010802027423号
京公网安备 11010802027423号