当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhancing SN38 prodrug delivery using a self-immolative linker and endogenous albumin transport
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-04-11 , DOI: 10.1016/j.jconrel.2024.04.019 Xing Jiang 1 , Lingyi Zhu 1 , Qingyu Wei 1 , Wei Lu 1 , Jiahui Yu 1 , Shulei Zhu 2
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-04-11 , DOI: 10.1016/j.jconrel.2024.04.019 Xing Jiang 1 , Lingyi Zhu 1 , Qingyu Wei 1 , Wei Lu 1 , Jiahui Yu 1 , Shulei Zhu 2
Affiliation
|
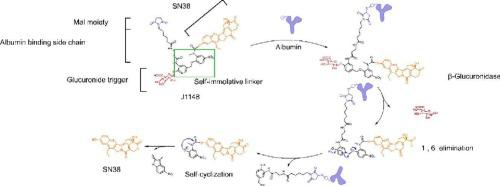
Enhancing the delivery and release efficiency of hydroxyl agents, constrained by high pK values and issues of release rate or unstable linkage, is a critical challenge. To address this, a self-immolative linker, composed of a modifiable p-hydroxybenzyl ether and a fast cyclization adapter (N-(ortho-hydroxyphenyl)--methylcarbamate) was strategically designed, for the synthesis of prodrugs. The innovative linker not only provides a side chain modification but also facilitates the rapid release of the active payloads, thereby enabling precise drug delivery. Particularly, five prodrug model compounds (J1, J2, J3, J5 and J6) were synthesized to evaluate the release rates by using β-glucuronic acid as trigger and five hydroxyl compounds as model payloads. Significantly, all prodrug model compounds could efficiently release the hydroxyl payloads under the action of β-glucuronidase, validating the robustness of the linker. And then, to assess the drug delivery and release efficiency using endogenous albumin as a transport vehicle, J1148, a SN38 prodrug modified with maleimide side chain was synthesized. Results demonstrated that J1148 covalently bound to plasma albumin through in situ Michael addition, effectively targeting the tumor microenvironment. Activated by β-glucuronidase, J1148 underwent a classical 1, 6-elimination, followed by rapid cyclization of the adapter, thereby releasing SN38. Impressively, J1148 showed excellent therapeutic efficacy against human colonic cancer xenograft model, leading to a significant reduction or even disappearance of tumors (3/6 of mice cured). These findings underscore the potential of the designed linker in the delivery system of hydroxyl agents, positioning it at the forefront of advancements in drug delivery technology.
中文翻译:

使用自毁连接子和内源性白蛋白运输增强 SN38 前药递送
由于高 pK 值以及释放速率或不稳定连接问题的限制,提高羟基制剂的递送和释放效率是一项严峻的挑战。为了解决这个问题,我们策略性地设计了一种由可修饰的对羟基苯甲基醚和快速环化接头(N-(邻羟基苯基)-甲基氨基甲酸酯)组成的自毁连接体,用于合成前药。创新的连接体不仅提供侧链修饰,而且有利于活性有效负载的快速释放,从而实现精确的药物递送。特别地,合成了五种前药模型化合物(J1、J2、J3、J5和J6),以β-葡萄糖醛酸作为触发剂和五种羟基化合物作为模型有效负载来评估释放速率。值得注意的是,所有前药模型化合物都可以在β-葡萄糖醛酸酶的作用下有效释放羟基有效负载,验证了连接体的稳健性。然后,为了评估使用内源性白蛋白作为转运载体的药物递送和释放效率,合成了马来酰亚胺侧链修饰的SN38前药J1148。结果表明,J1148 通过原位迈克尔加成与血浆白蛋白共价结合,有效靶向肿瘤微环境。在 β-葡萄糖醛酸酶的激活下,J1148 经历了经典的 1, 6-消除,随后接头快速环化,从而释放 SN38。令人印象深刻的是,J1148对人结肠癌异种移植模型表现出优异的治疗效果,导致肿瘤显着减少甚至消失(3/6的小鼠治愈)。这些发现强调了所设计的连接体在羟基药物递送系统中的潜力,使其处于药物递送技术进步的前沿。
更新日期:2024-04-11
中文翻译:

使用自毁连接子和内源性白蛋白运输增强 SN38 前药递送
由于高 pK 值以及释放速率或不稳定连接问题的限制,提高羟基制剂的递送和释放效率是一项严峻的挑战。为了解决这个问题,我们策略性地设计了一种由可修饰的对羟基苯甲基醚和快速环化接头(N-(邻羟基苯基)-甲基氨基甲酸酯)组成的自毁连接体,用于合成前药。创新的连接体不仅提供侧链修饰,而且有利于活性有效负载的快速释放,从而实现精确的药物递送。特别地,合成了五种前药模型化合物(J1、J2、J3、J5和J6),以β-葡萄糖醛酸作为触发剂和五种羟基化合物作为模型有效负载来评估释放速率。值得注意的是,所有前药模型化合物都可以在β-葡萄糖醛酸酶的作用下有效释放羟基有效负载,验证了连接体的稳健性。然后,为了评估使用内源性白蛋白作为转运载体的药物递送和释放效率,合成了马来酰亚胺侧链修饰的SN38前药J1148。结果表明,J1148 通过原位迈克尔加成与血浆白蛋白共价结合,有效靶向肿瘤微环境。在 β-葡萄糖醛酸酶的激活下,J1148 经历了经典的 1, 6-消除,随后接头快速环化,从而释放 SN38。令人印象深刻的是,J1148对人结肠癌异种移植模型表现出优异的治疗效果,导致肿瘤显着减少甚至消失(3/6的小鼠治愈)。这些发现强调了所设计的连接体在羟基药物递送系统中的潜力,使其处于药物递送技术进步的前沿。











































 京公网安备 11010802027423号
京公网安备 11010802027423号