Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Gallic acid-loaded HFZIF-8 for tumor-targeted delivery and thermal-catalytic therapy
Nanoscale ( IF 6.7 ) Pub Date : 2024-04-11 , DOI: 10.1039/d4nr01102c Xing Yang , Chunsheng Li , Shuang Liu , Yunlong Li , Xinyu Zhang , Qiang Wang , Jin Ye , Yong Lu , Yujie Fu , Jiating Xu
Nanoscale ( IF 6.7 ) Pub Date : 2024-04-11 , DOI: 10.1039/d4nr01102c Xing Yang , Chunsheng Li , Shuang Liu , Yunlong Li , Xinyu Zhang , Qiang Wang , Jin Ye , Yong Lu , Yujie Fu , Jiating Xu
|
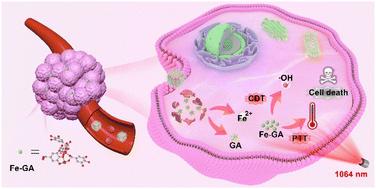
“Transition” metal-coordinated plant polyphenols are a type of promising antitumor nanodrugs owing to their high biosafety and catalytic therapy potency; however, the major obstacle restricting their clinical application is their poor tumor accumulation. Herein, Fe-doped ZIF-8 was tailored using tannic acid (TA) into a hollow mesoporous nanocarrier for gallic acid (GA) loading. After hyaluronic acid (HA) modification, the developed nanosystem of HFZIF-8/GA@HA was used for the targeted delivery of Fe ions and GA, thereby intratumorally achieving the synthesis of an Fe-GA coordinated complex. The TA-etching strategy facilitated the development of a cavitary structure and abundant coordination sites of ZIF-8, thus ensuring an ideal loading efficacy of GA (23.4 wt%). When HFZIF-8/GA@HA accumulates in the tumor microenvironment (TME), the framework is broken due to the competitive protonation ability of overexpressed protons in the TME. Interestingly, the intratumoral degradation of HFZIF-8/GA@HA provides the opportunity for the in situ “meeting” of GA and Fe ions, and through the coordination of polyhydroxyls assisted by conjugated electrons on the benzene ring, highly stable Fe-GA nanochelates are formed. Significantly, owing to the electron delocalization effect of GA, intratumorally coordinated Fe-GA could efficiently absorb second near-infrared (NIR-II, 1064 nm) laser irradiation and transfer it into thermal energy with a conversion efficiency of 36.7%. The photothermal performance could speed up the Fenton reaction rate of Fe-GA with endogenous H2O2 for generating more hydroxyl radicals, thus realizing thermally enhanced chemodynamic therapy. Overall, our research findings demonstrate that HFZIF-8/GA@HA has potential as a safe and efficient anticancer nanodrug.
中文翻译:

负载没食子酸的 HFZIF-8 用于肿瘤靶向递送和热催化治疗
“过渡”金属配位植物多酚由于其高生物安全性和催化治疗效力,是一种有前途的抗肿瘤纳米药物;然而,限制其临床应用的主要障碍是其肿瘤积累能力差。在此,使用单宁酸(TA)将铁掺杂的ZIF-8定制成空心介孔纳米载体,用于负载没食子酸(GA)。经过透明质酸(HA)修饰后,开发的HFZIF-8/GA@HA纳米系统用于靶向递送Fe离子和GA,从而在瘤内实现Fe-GA配位复合物的合成。 TA蚀刻策略促进了ZIF-8空穴结构和丰富配位位点的发展,从而确保了GA理想的负载效率(23.4 wt%)。当 HFZIF-8/GA@HA 在肿瘤微环境 (TME) 中积累时,由于 TME 中过度表达的质子的竞争性质子化能力,框架被破坏。有趣的是,HFZIF-8/GA@HA的瘤内降解为GA和Fe离子的原位“相遇”提供了机会,并通过苯环上共轭电子辅助的多羟基配位,形成高度稳定的Fe-GA纳米螯合物形成。值得注意的是,由于GA的电子离域效应,瘤内配位的Fe-GA可以有效吸收第二次近红外(NIR-II,1064 nm)激光照射并将其转化为热能,转换效率为36.7%。光热性能可以加快Fe-GA与内源性H 2 O 2的Fenton反应速率,产生更多的羟基自由基,从而实现热强化化学动力学治疗。总体而言,我们的研究结果表明 HFZIF-8/GA@HA 具有作为安全有效的抗癌纳米药物的潜力。
更新日期:2024-04-11
中文翻译:

负载没食子酸的 HFZIF-8 用于肿瘤靶向递送和热催化治疗
“过渡”金属配位植物多酚由于其高生物安全性和催化治疗效力,是一种有前途的抗肿瘤纳米药物;然而,限制其临床应用的主要障碍是其肿瘤积累能力差。在此,使用单宁酸(TA)将铁掺杂的ZIF-8定制成空心介孔纳米载体,用于负载没食子酸(GA)。经过透明质酸(HA)修饰后,开发的HFZIF-8/GA@HA纳米系统用于靶向递送Fe离子和GA,从而在瘤内实现Fe-GA配位复合物的合成。 TA蚀刻策略促进了ZIF-8空穴结构和丰富配位位点的发展,从而确保了GA理想的负载效率(23.4 wt%)。当 HFZIF-8/GA@HA 在肿瘤微环境 (TME) 中积累时,由于 TME 中过度表达的质子的竞争性质子化能力,框架被破坏。有趣的是,HFZIF-8/GA@HA的瘤内降解为GA和Fe离子的原位“相遇”提供了机会,并通过苯环上共轭电子辅助的多羟基配位,形成高度稳定的Fe-GA纳米螯合物形成。值得注意的是,由于GA的电子离域效应,瘤内配位的Fe-GA可以有效吸收第二次近红外(NIR-II,1064 nm)激光照射并将其转化为热能,转换效率为36.7%。光热性能可以加快Fe-GA与内源性H 2 O 2的Fenton反应速率,产生更多的羟基自由基,从而实现热强化化学动力学治疗。总体而言,我们的研究结果表明 HFZIF-8/GA@HA 具有作为安全有效的抗癌纳米药物的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号