当前位置:
X-MOL 学术
›
Catal. Today
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
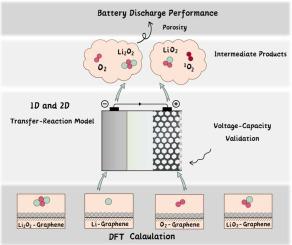
Elucidate the formation and consumption mechanism of lithium oxides in lithium-oxygen batteries by combining the transfer-reaction model and the DFT calculation
Catalysis Today ( IF 5.3 ) Pub Date : 2024-04-03 , DOI: 10.1016/j.cattod.2024.114700 Jiadong Wu , Jia-Hui Li , Honglai Liu , Cheng Lian
Catalysis Today ( IF 5.3 ) Pub Date : 2024-04-03 , DOI: 10.1016/j.cattod.2024.114700 Jiadong Wu , Jia-Hui Li , Honglai Liu , Cheng Lian
|
Lithium-oxygen (Li-O) batteries are considered one of the most promising energy storage devices for the next generation due to their high theoretical energy density. However, its poor cycle efficiency and discharge performance hinder the large-scale application. Understanding the complicative positive electrode reaction of Li-O batteries can help overcome these difficulties. This work establishes a transfer-reaction model to simulate the multi-step reactions during the discharge of Li-O batteries. It is found that the internal diffusion of oxygen causes local deposition of LiO at the interface, leading to deterioration of diffusion and the end of discharge. The intermediate product LiO accumulates uniformly through the electrode, while the generated singlet oxygen exhibits a characteristic of less at the interface and more inside. By using DFT to calculate the reaction rate constant, the model is proved to achieve the connection between macro and micro levels well. This work hopes to inspire future multi-scale research into Li-O batteries and contribute to the realization of high-performance metal-air battery design as soon as possible.
中文翻译:

结合转移反应模型和DFT计算阐明锂氧电池中锂氧化物的形成和消耗机制
锂氧(Li-O)电池由于其高理论能量密度而被认为是下一代最有前途的储能设备之一。但其循环效率和放电性能较差阻碍了其大规模应用。了解锂氧电池复杂的正极反应有助于克服这些困难。这项工作建立了转移反应模型来模拟锂氧电池放电过程中的多步反应。研究发现,氧的内部扩散导致LiO在界面处局部沉积,导致扩散恶化和放电终止。中间产物Li2O通过电极均匀积累,而生成的单线态氧呈现出界面少、内部多的特点。通过使用DFT计算反应速率常数,证明该模型很好地实现了宏观和微观层面的联系。这项工作希望能够激发未来对锂氧电池的多尺度研究,并为尽快实现高性能金属空气电池设计做出贡献。
更新日期:2024-04-03
中文翻译:

结合转移反应模型和DFT计算阐明锂氧电池中锂氧化物的形成和消耗机制
锂氧(Li-O)电池由于其高理论能量密度而被认为是下一代最有前途的储能设备之一。但其循环效率和放电性能较差阻碍了其大规模应用。了解锂氧电池复杂的正极反应有助于克服这些困难。这项工作建立了转移反应模型来模拟锂氧电池放电过程中的多步反应。研究发现,氧的内部扩散导致LiO在界面处局部沉积,导致扩散恶化和放电终止。中间产物Li2O通过电极均匀积累,而生成的单线态氧呈现出界面少、内部多的特点。通过使用DFT计算反应速率常数,证明该模型很好地实现了宏观和微观层面的联系。这项工作希望能够激发未来对锂氧电池的多尺度研究,并为尽快实现高性能金属空气电池设计做出贡献。



























 京公网安备 11010802027423号
京公网安备 11010802027423号