当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photochemistry of CH3I⋯(H2O)n Complexes: From CH3I⋯H2O to CH3I in Interaction with Water Ices and Atmospheric Implications
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2024-04-11 , DOI: 10.1021/acsearthspacechem.3c00351 Sophie Sobanska 1 , Michelle T. Custodio-Castro 2 , Rosana M. Romano 2 , Joëlle Mascetti 1 , Stéphane Coussan 3
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2024-04-11 , DOI: 10.1021/acsearthspacechem.3c00351 Sophie Sobanska 1 , Michelle T. Custodio-Castro 2 , Rosana M. Romano 2 , Joëlle Mascetti 1 , Stéphane Coussan 3
Affiliation
|
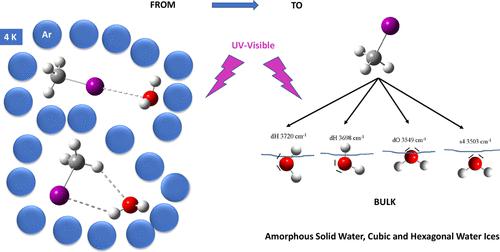
The interaction of methyl iodine with the surface of amorphous, cubic, and hexagonal ices has been investigated. The CH3I desorption process has also been evaluated. We have highlighted the difference in CH3I behavior depending on the trapping on the ice surface. The broadband UV photochemistry of CH3I trapped at the surface of ices has been studied. Those results have been compared with UV broadband photochemistry of CH3I bare monomer complexed with water and trapped in argon cryogenic matrices. It appears that if CH3I interacts with water molecules or water ice by hydrogen bonding, then CH3I does not fragment under UV irradiation. Thus, energy transfer to the network of hydrogen bonds in the ice or matrix is effective. On the other hand, to a first approximation, if CH3I interacts by picnogen-type bonding (I⋯O), then CH3I fragments, because the electronic relaxation seems to take place mainly at the intramolecular levels of CH3I. Finally, we demonstrated that water ice does not catalyze the photofragmentation of CH3I. Rather, it modifies the electronic relaxation paths, some of which lead to the fragmentation of iodomethane. This fundamental work provides an understanding of the molecular processes involved in water/ice–CH3I interaction and the role of these molecular interactions on CH3I photochemistry in the atmosphere.
中文翻译:

CH3I⋯(H2O)n 配合物的光化学:从 CH3I⋯H2O 到 CH3I 与水冰的相互作用以及对大气的影响
研究了甲基碘与无定形冰、立方冰和六角冰表面的相互作用。 CH 3 I 解吸过程也得到了评估。我们强调了 CH 3 I 行为的差异取决于冰表面的捕获。研究了冰表面捕获的CH 3 I的宽带紫外光化学。这些结果已与与水络合并捕获在氩低温基质中的CH 3 I 裸单体的紫外宽带光化学进行了比较。看来如果CH 3 I通过氢键与水分子或水冰相互作用,则CH 3 I在紫外线照射下不会碎裂。因此,能量转移到冰或基质中的氢键网络是有效的。另一方面,对于第一近似,如果 CH 3 I 通过 picnogen 型键合 (I⋯O) 相互作用,则 CH 3 I 断裂,因为电子弛豫似乎主要发生在 CH 3 I的分子内水平。最后,我们证明水冰不会催化CH 3 I的光裂解。相反,它会改变电子弛豫路径,其中一些导致碘甲烷的裂解。这项基础工作提供了对水/冰-CH 3 I相互作用所涉及的分子过程以及这些分子相互作用对大气中CH 3 I光化学的作用的理解。
更新日期:2024-04-11
中文翻译:

CH3I⋯(H2O)n 配合物的光化学:从 CH3I⋯H2O 到 CH3I 与水冰的相互作用以及对大气的影响
研究了甲基碘与无定形冰、立方冰和六角冰表面的相互作用。 CH 3 I 解吸过程也得到了评估。我们强调了 CH 3 I 行为的差异取决于冰表面的捕获。研究了冰表面捕获的CH 3 I的宽带紫外光化学。这些结果已与与水络合并捕获在氩低温基质中的CH 3 I 裸单体的紫外宽带光化学进行了比较。看来如果CH 3 I通过氢键与水分子或水冰相互作用,则CH 3 I在紫外线照射下不会碎裂。因此,能量转移到冰或基质中的氢键网络是有效的。另一方面,对于第一近似,如果 CH 3 I 通过 picnogen 型键合 (I⋯O) 相互作用,则 CH 3 I 断裂,因为电子弛豫似乎主要发生在 CH 3 I的分子内水平。最后,我们证明水冰不会催化CH 3 I的光裂解。相反,它会改变电子弛豫路径,其中一些导致碘甲烷的裂解。这项基础工作提供了对水/冰-CH 3 I相互作用所涉及的分子过程以及这些分子相互作用对大气中CH 3 I光化学的作用的理解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号