Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Targeting the MCP‐GPX4/HMGB1 Axis for Effectively Triggering Immunogenic Ferroptosis in Pancreatic Ductal Adenocarcinoma
Advanced Science ( IF 15.1 ) Pub Date : 2024-04-09 , DOI: 10.1002/advs.202308208 Ge Li 1 , Chengyu Liao 2 , Jiangzhi Chen 1 , Zuwei Wang 2, 3 , Shuncang Zhu 2 , Jianlin Lai 2, 3 , Qiaowei Li 4, 5 , Yinhao Chen 2 , Dihan Wu 2 , Jianbo Li 2 , Yi Huang 2, 6 , Yifeng Tian 2, 3 , Yanling Chen 1 , Shi Chen 2, 3, 4, 5
Advanced Science ( IF 15.1 ) Pub Date : 2024-04-09 , DOI: 10.1002/advs.202308208 Ge Li 1 , Chengyu Liao 2 , Jiangzhi Chen 1 , Zuwei Wang 2, 3 , Shuncang Zhu 2 , Jianlin Lai 2, 3 , Qiaowei Li 4, 5 , Yinhao Chen 2 , Dihan Wu 2 , Jianbo Li 2 , Yi Huang 2, 6 , Yifeng Tian 2, 3 , Yanling Chen 1 , Shi Chen 2, 3, 4, 5
Affiliation
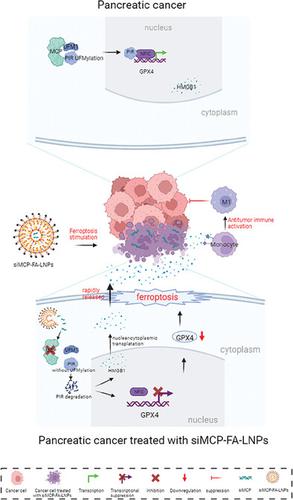
|
Induction of ferroptosis can inhibit cancer cells in vitro, however, the role of ferroptosis in treatment in vivo is controversial. The immunosuppressive cells activated by the ferroptotic tumor cells can promote the growth of residual tumor cells, hindering the application of ferroptosis stimulation in tumor treatment. In this study, a new strategy is aimed to be identified for effectively triggering immunogenic ferroptosis in pancreatic ductal adenocarcinoma (PDAC) and simultaneously stimulating antitumor immune responses. Toward this, several molecular and biochemical experiments are performed using patient‐derived organoid models and a KPC mouse model (LSL ‐KrasG12D /+ , LSL‐Trp53R172H/+ , Pdx‐1‐Cre ). It is observed that the inhibition of macrophage‐capping protein (MCP) suppressed the ubiquitin fold modifier (UFM)ylation of pirin (PIR), a newly identified substrate of UFM1, thereby decreasing the transcription of GPX4 , a marker of ferroptosis, and promoting the cytoplasmic transportation of HMGB1, a damage‐associated molecular pattern. GPX4 deficiency triggered ferroptosis, and the pre‐accumulated cytosolic HMGB1 is released rapidly. This altered release pattern of HMGB1 facilitated the pro‐inflammatory M1‐like polarization of macrophages. Thus, therapeutic inhibition of MCP yielded dual antitumor effects by stimulating ferroptosis and activating antitumor pro‐inflammatory M1‐like macrophages. The nanosystem developed for specifically silencing MCP is a promising tool for treating PDAC.
中文翻译:

靶向 MCP-GPX4/HMGB1 轴有效触发胰腺导管腺癌中的免疫原性铁死亡
诱导铁死亡可以在体外抑制癌细胞,然而,铁死亡在体内治疗中的作用存在争议。铁死亡肿瘤细胞激活的免疫抑制细胞可以促进残留肿瘤细胞的生长,阻碍铁死亡刺激在肿瘤治疗中的应用。在本研究中,旨在确定一种新策略,用于有效触发胰腺导管腺癌(PDAC)中的免疫原性铁死亡,同时刺激抗肿瘤免疫反应。为此,使用患者来源的类器官模型和 KPC 小鼠模型进行了多项分子和生化实验(LSL ‐克拉斯G12D /+ ,LSL-Trp53R172H/+ ,Pdx-1-Cre )。据观察,巨噬细胞加帽蛋白 (MCP) 的抑制抑制了 pirin (PIR)(一种新鉴定的 UFM1 底物)的泛素折叠修饰剂 (UFM) 化,从而减少了GPX4 ,铁死亡的标志物,并促进 HMGB1(一种损伤相关分子模式)的细胞质运输。GPX4 缺乏会引发铁死亡,预先积累的胞质 HMGB1 会迅速释放。 HMGB1 释放模式的改变促进了巨噬细胞的促炎性 M1 样极化。因此,治疗性抑制MCP 通过刺激铁死亡和激活抗肿瘤促炎性 M1 样巨噬细胞产生双重抗肿瘤作用。专为沉默而开发的纳米系统MCP 是一种很有前景的治疗 PDAC 的工具。
更新日期:2024-04-09
中文翻译:

靶向 MCP-GPX4/HMGB1 轴有效触发胰腺导管腺癌中的免疫原性铁死亡
诱导铁死亡可以在体外抑制癌细胞,然而,铁死亡在体内治疗中的作用存在争议。铁死亡肿瘤细胞激活的免疫抑制细胞可以促进残留肿瘤细胞的生长,阻碍铁死亡刺激在肿瘤治疗中的应用。在本研究中,旨在确定一种新策略,用于有效触发胰腺导管腺癌(PDAC)中的免疫原性铁死亡,同时刺激抗肿瘤免疫反应。为此,使用患者来源的类器官模型和 KPC 小鼠模型进行了多项分子和生化实验(



























 京公网安备 11010802027423号
京公网安备 11010802027423号